Origin of twist-bend coupling in actin filaments
- PMID: 20858430
- PMCID: PMC2941021
- DOI: 10.1016/j.bpj.2010.07.009
Origin of twist-bend coupling in actin filaments
Abstract
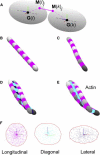
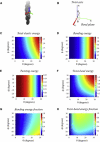
Actin filaments are semiflexible polymers that display large-scale conformational twisting and bending motions. Modulation of filament bending and twisting dynamics has been linked to regulatory actin-binding protein function, filament assembly and fragmentation, and overall cell motility. The relationship between actin filament bending and twisting dynamics has not been evaluated. The numerical and analytical experiments presented here reveal that actin filaments have a strong intrinsic twist-bend coupling that obligates the reciprocal interconversion of bending energy and twisting stress. We developed a mesoscopic model of actin filaments that captures key documented features, including the subunit dimensions, interaction energies, helicity, and geometrical constraints coming from the double-stranded structure. The filament bending and torsional rigidities predicted by the model are comparable to experimental values, demonstrating the capacity of the model to assess the mechanical properties of actin filaments, including the coupling between twisting and bending motions. The predicted actin filament twist-bend coupling is strong, with a persistence length of 0.15-0.4 μm depending on the actin-bound nucleotide. Twist-bend coupling is an emergent property that introduces local asymmetry to actin filaments and contributes to their overall elasticity. Up to 60% of the filament subunit elastic free energy originates from twist-bend coupling, with the largest contributions resulting under relatively small deformations. A comparison of filaments with different architectures indicates that twist-bend coupling in actin filaments originates from their double protofilament and helical structure.
Copyright © 2010 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Biophysics of actin filament severing by cofilin.FEBS Lett. 2013 Apr 17;587(8):1215-9. doi: 10.1016/j.febslet.2013.01.062. Epub 2013 Feb 5. FEBS Lett. 2013. PMID: 23395798 Free PMC article. Review.
-
Mechanical heterogeneity favors fragmentation of strained actin filaments.Biophys J. 2015 May 5;108(9):2270-81. doi: 10.1016/j.bpj.2015.03.058. Biophys J. 2015. PMID: 25954884 Free PMC article.
-
Twist response of actin filaments.Proc Natl Acad Sci U S A. 2023 Jan 24;120(4):e2208536120. doi: 10.1073/pnas.2208536120. Epub 2023 Jan 19. Proc Natl Acad Sci U S A. 2023. PMID: 36656858 Free PMC article.
-
Actin Filament Strain Promotes Severing and Cofilin Dissociation.Biophys J. 2017 Jun 20;112(12):2624-2633. doi: 10.1016/j.bpj.2017.05.016. Biophys J. 2017. PMID: 28636918 Free PMC article.
-
The many implications of actin filament helicity.Semin Cell Dev Biol. 2020 Jun;102:65-72. doi: 10.1016/j.semcdb.2019.10.018. Epub 2019 Dec 18. Semin Cell Dev Biol. 2020. PMID: 31862222 Review.
Cited by
-
Biophysics of actin filament severing by cofilin.FEBS Lett. 2013 Apr 17;587(8):1215-9. doi: 10.1016/j.febslet.2013.01.062. Epub 2013 Feb 5. FEBS Lett. 2013. PMID: 23395798 Free PMC article. Review.
-
Actin age orchestrates myosin-5 and myosin-6 run lengths.Curr Biol. 2015 Aug 3;25(15):2057-62. doi: 10.1016/j.cub.2015.06.033. Epub 2015 Jul 16. Curr Biol. 2015. PMID: 26190073 Free PMC article.
-
Clusters of a Few Bound Cofilins Sever Actin Filaments.J Mol Biol. 2021 Apr 2;433(7):166833. doi: 10.1016/j.jmb.2021.166833. Epub 2021 Jan 30. J Mol Biol. 2021. PMID: 33524412 Free PMC article.
-
Insights into the Cooperative Nature of ATP Hydrolysis in Actin Filaments.Biophys J. 2018 Oct 16;115(8):1589-1602. doi: 10.1016/j.bpj.2018.08.034. Epub 2018 Sep 1. Biophys J. 2018. PMID: 30249402 Free PMC article.
-
Dynamin rings: not just for fission.Traffic. 2013 Dec;14(12):1194-9. doi: 10.1111/tra.12116. Epub 2013 Sep 19. Traffic. 2013. PMID: 23980695 Free PMC article. Review.
References
-
- Holmes K.C., Popp D., Kabsch W. Atomic model of the actin filament. Nature. 1990;347:44–49. - PubMed
-
- Isambert H., Venier P., Carlier M.F. Flexibility of actin filaments derived from thermal fluctuations. Effect of bound nucleotide, phalloidin, and muscle regulatory proteins. J. Biol. Chem. 1995;270:11437–11444. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

