Development and applications of photo-triggered theranostic agents
- PMID: 20858520
- PMCID: PMC2991599
- DOI: 10.1016/j.addr.2010.09.002
Development and applications of photo-triggered theranostic agents
Abstract
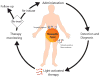
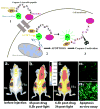
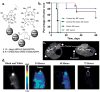
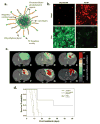
Theranostics, the fusion of therapy and diagnostics for optimizing efficacy and safety of therapeutic regimes, is a growing field that is paving the way towards the goal of personalized medicine for the benefit of patients. The use of light as a remote-activation mechanism for drug delivery has received increased attention due to its advantages in highly specific spatial and temporal control of compound release. Photo-triggered theranostic constructs could facilitate an entirely new category of clinical solutions which permit early recognition of the disease by enhancing contrast in various imaging modalities followed by the tailored guidance of therapy. Finally, such theranostic agents could aid imaging modalities in monitoring response to therapy. This article reviews recent developments in the use of light-triggered theranostic agents for simultaneous imaging and photoactivation of therapeutic agents. Specifically, we discuss recent developments in the use of theranostic agents for photodynamic-, photothermal- or photo-triggered chemotherapy for several diseases.
Published by Elsevier B.V.
Figures




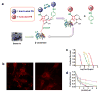
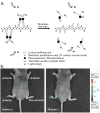
References
-
-
Rising Health Costs a Global Problem, in, 2010.
-
-
- WORLD HEALTH STATISTICS 2010. World Health Organization; France: 2010. pp. 1–177.
-
- Rai AJ, Yee J, Fleisher M. Biomarkers in the era of personalized medicine - a multiplexed SNP assay using capillary electrophoresis for assessing drug metabolism capacity. Scand J Clin Lab Invest Suppl. 2010;242:15–18. - PubMed
-
- Blanchet KD. Redefining personalized medicine in the postgenomic era: developing bladder cancer therapeutics with proteomics. BJU Int. 2010;105:i–iii. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

