Functional analysis of two single nucleotide polymorphisms in SLC30A2 (ZnT2): implications for mammary gland function and breast disease in women
- PMID: 20858712
- PMCID: PMC3008367
- DOI: 10.1152/physiolgenomics.00137.2010
Functional analysis of two single nucleotide polymorphisms in SLC30A2 (ZnT2): implications for mammary gland function and breast disease in women
Abstract
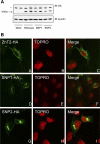
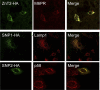
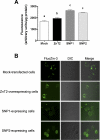
Zinc transporter 2 (ZnT2) plays a major role in zinc (Zn) export from the mammary gland. Recently, we determined that ZnT2 is associated with secretory vesicles reflecting its role in Zn secretion during lactation. Herein, we identified two distinct single nucleotide polymorphisms (SNPs) in SLC30A2, which encodes ZnT2. SNP1 (rs35235055) results in a leucine-to-proline substitution (Leu(23)Pro), while SNP2 (rs35623192) results in an arginine-to-cysteine substitution (Arg(340)Cys). We examined the localization and function of each SNP in cells generated to express these polymorphic variants. SNP1 was mislocalized to lysosomes, while SNP2 was mislocalized to the Golgi apparatus. FluoZin-3 fluorescence illustrated increased lysosomal accumulation of Zn in cells expressing SNP1 concomitant with the abrogation of Zn secretion. In contrast, ectopic expression of SNP2 was associated with the expansion of cytoplasmic Zn pools, elevated reactive oxygen species, and increased Zn efflux. Taken together, our data indicate that polymorphic variants in ZnT2 distinctly alter mammary cell Zn metabolism. We speculate that these SNPs may compromise mammary cell function, which may have important implications in human health and breast disease.
Figures


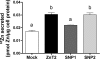
Similar articles
-
Exome Sequencing of SLC30A2 Identifies Novel Loss- and Gain-of-Function Variants Associated with Breast Cell Dysfunction.J Mammary Gland Biol Neoplasia. 2015 Dec;20(3-4):159-72. doi: 10.1007/s10911-015-9338-z. Epub 2015 Aug 21. J Mammary Gland Biol Neoplasia. 2015. PMID: 26293594
-
A genetic variant in SLC30A2 causes breast dysfunction during lactation by inducing ER stress, oxidative stress and epithelial barrier defects.Sci Rep. 2018 Feb 23;8(1):3542. doi: 10.1038/s41598-018-21505-8. Sci Rep. 2018. PMID: 29476070 Free PMC article.
-
A common genetic variant in zinc transporter ZnT2 (Thr288Ser) is present in women with low milk volume and alters lysosome function and cell energetics.Am J Physiol Cell Physiol. 2020 Jun 1;318(6):C1166-C1177. doi: 10.1152/ajpcell.00383.2019. Epub 2020 Apr 22. Am J Physiol Cell Physiol. 2020. PMID: 32320289
-
The role of the zinc transporter SLC30A2/ZnT2 in transient neonatal zinc deficiency.Metallomics. 2017 Oct 18;9(10):1352-1366. doi: 10.1039/c7mt00162b. Metallomics. 2017. PMID: 28665435 Review.
-
Zinc in specialized secretory tissues: roles in the pancreas, prostate, and mammary gland.Adv Nutr. 2011 Mar;2(2):101-11. doi: 10.3945/an.110.000232. Epub 2011 Mar 10. Adv Nutr. 2011. PMID: 22332039 Free PMC article. Review.
Cited by
-
A dominant negative heterozygous G87R mutation in the zinc transporter, ZnT-2 (SLC30A2), results in transient neonatal zinc deficiency.J Biol Chem. 2012 Aug 24;287(35):29348-61. doi: 10.1074/jbc.M112.368159. Epub 2012 Jun 25. J Biol Chem. 2012. PMID: 22733820 Free PMC article. Clinical Trial.
-
Dissecting the Process of Activation of Cancer-promoting Zinc-requiring Ectoenzymes by Zinc Metalation Mediated by ZNT Transporters.J Biol Chem. 2017 Feb 10;292(6):2159-2173. doi: 10.1074/jbc.M116.763946. Epub 2016 Dec 27. J Biol Chem. 2017. PMID: 28028180 Free PMC article.
-
Transition metals activate TFEB in overexpressing cells.Biochem J. 2015 Aug 15;470(1):65-76. doi: 10.1042/BJ20140645. Epub 2015 Jun 11. Biochem J. 2015. PMID: 26251447 Free PMC article.
-
Paradoxical zinc toxicity and oxidative stress in the mammary gland during marginal dietary zinc deficiency.Reprod Toxicol. 2015 Jul;54:84-92. doi: 10.1016/j.reprotox.2014.07.076. Epub 2014 Aug 1. Reprod Toxicol. 2015. PMID: 25088245 Free PMC article.
-
Genetic and Physiological Factors Affecting Human Milk Production and Composition.Nutrients. 2020 May 21;12(5):1500. doi: 10.3390/nu12051500. Nutrients. 2020. PMID: 32455695 Free PMC article. Review.
References
-
- Atherton DJ, Muller DP, Aggett PJ, Harries JT. A defect in zinc uptake by jejunal biopsies in acrodermatitis enteropathica. Clin Sci (Lond) 56: 505–507, 1979 - PubMed
-
- Bag A, Bag N. Target sequence polymorphism of human manganese superoxide dismutase gene and its association with cancer risk: a review. Cancer Epidemiol Biomarkers Prev 17: 3298–3305, 2008 - PubMed
-
- Bolte S, Cordelieres FP. A guided tour into subcellular colocalization analysis in light microscopy. J Microsc 224: 213–232, 2006 - PubMed
-
- Chen HJ, Yuan J, Lobel P. Systematic mutational analysis of the cation-independent mannose 6-phosphate/insulin-like growth factor II receptor cytoplasmic domain. An acidic cluster containing a key aspartate is important for function in lysosomal enzyme sorting. J Biol Chem 272: 7003–7012, 1997 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

