The use of one-bead one-compound combinatorial library technology to discover high-affinity αvβ3 integrin and cancer targeting arginine-glycine-aspartic acid ligands with a built-in handle
- PMID: 20858725
- PMCID: PMC3571112
- DOI: 10.1158/1535-7163.MCT-10-0308
The use of one-bead one-compound combinatorial library technology to discover high-affinity αvβ3 integrin and cancer targeting arginine-glycine-aspartic acid ligands with a built-in handle
Abstract
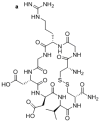
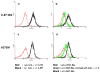
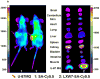
The αvβ3 integrin, expressed on the surface of various normal and cancer cells, is involved in numerous physiologic processes such as angiogenesis, apoptosis, and bone resorption. Because this integrin plays a key role in angiogenesis and metastasis of human tumors, αvβ3 integrin ligands are of great interest to advances in targeted therapy and cancer imaging. In this report, one-bead one-compound (OBOC) combinatorial libraries containing the arginine-glycine-aspartic acid (RGD) motif were designed and screened against K562 myeloid leukemia cells that had been transfected with the human αvβ3 integrin gene. Cyclic peptide LXW7 was identified as a leading ligand with a built-in handle that binds specifically to αvβ3 and showed comparable binding affinity (IC(50) = 0.68 ± 0.08 μmol/L) to some of the well-known RGD "head-to-tail" cyclic pentapeptide ligands reported in the literature. The biotinylated form of LXW7 ligand showed similar binding strength as LXW7 against αvβ3 integrin, whereas biotinylated RGD cyclopentapeptide ligands revealed a 2- to 8-fold weaker binding affinity than their free forms. LXW7 was able to bind to both U-87MG glioblastoma and A375M melanoma cell lines, both of which express high levels of αvβ3 integrin. In vivo and ex vivo optical imaging studies with the biotinylated ligand/streptavidin-Cy5.5 complex in nude mice bearing U-87MG or A375M xenografts revealed preferential uptake of biotinylated LXW7 in tumor. When compared with biotinylated RGD cyclopentapeptide ligands, biotinylated LXW7 showed higher tumor uptake but lower liver uptake.
Conflict of interest statement
There are no potential conflicts of interest.
Figures






Similar articles
-
Optimization of RGD-Containing Cyclic Peptides against αvβ3 Integrin.Mol Cancer Ther. 2016 Feb;15(2):232-40. doi: 10.1158/1535-7163.MCT-15-0544. Epub 2015 Dec 30. Mol Cancer Ther. 2016. PMID: 26719578 Free PMC article.
-
Self-quenched-regioselectively addressable functionalized template-[cyclo-(RGD-d-Phe-Lys)]4 peptide-Cy5-fluorescence quencher QSY21.2007 Oct 31 [updated 2008 Jan 21]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2007 Oct 31 [updated 2008 Jan 21]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 20641805 Free Books & Documents. Review.
-
Cy5-Regioselectively addressable functionalized template-[cyclo-(RGD-d-Phe-Lys)]4 peptide.2007 Oct 9 [updated 2007 Dec 28]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2007 Oct 9 [updated 2007 Dec 28]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 20641556 Free Books & Documents. Review.
-
131I-Labeled arginine-arginine-leucine (RRL)-containing cyclic peptide (YCGGRRLGGC) for imaging prostate carcinoma.2010 Jan 6 [updated 2010 Feb 16]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2010 Jan 6 [updated 2010 Feb 16]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 20641373 Free Books & Documents. Review.
-
Ligands for mapping alphavbeta3-integrin expression in vivo.Acc Chem Res. 2009 Jul 21;42(7):969-80. doi: 10.1021/ar800243b. Acc Chem Res. 2009. PMID: 19489579
Cited by
-
Microscale radiosynthesis, preclinical imaging and dosimetry study of [18F]AMBF3-TATE: A potential PET tracer for clinical imaging of somatostatin receptors.Nucl Med Biol. 2018 Jun;61:36-44. doi: 10.1016/j.nucmedbio.2018.04.001. Epub 2018 Apr 20. Nucl Med Biol. 2018. PMID: 29747035 Free PMC article.
-
GHGKHKNK octapeptide (P-5m) inhibits metastasis of HCCLM3 cell lines via regulation of MMP-2 expression in in vitro and in vivo studies.Molecules. 2012 Feb 2;17(2):1357-72. doi: 10.3390/molecules17021357. Molecules. 2012. PMID: 22395332 Free PMC article.
-
Construction of tissue-engineered vascular grafts with enhanced patency by integrating heparin, cell-adhesive peptide, and carbon monoxide nanogenerators into acellular blood vessels.Bioact Mater. 2023 Dec 28;34:221-236. doi: 10.1016/j.bioactmat.2023.12.015. eCollection 2024 Apr. Bioact Mater. 2023. PMID: 38235307 Free PMC article.
-
Design, synthesis, and application of OB2C combinatorial peptide and peptidomimetic libraries.Methods Mol Biol. 2015;1248:3-22. doi: 10.1007/978-1-4939-2020-4_1. Methods Mol Biol. 2015. PMID: 25616322 Free PMC article. Review.
-
Combinatorial peptide libraries: mining for cell-binding peptides.Chem Rev. 2014 Jan 22;114(2):1020-81. doi: 10.1021/cr400166n. Epub 2013 Dec 3. Chem Rev. 2014. PMID: 24299061 Free PMC article. Review. No abstract available.
References
-
- Pierschbacher MD, Ruoslahti E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature. 1984;309:30–3. - PubMed
-
- Haubner R, Gratias R, Diefenbach B, Goodman SL, Jonczyk A, Kessler H. Structural and functional aspects of RGD-containing cyclic pentapeptides as highly potent and selective integrin αvβ3 antagonists. J Am Chem Soc. 1996;118:13.
-
- Markland FS. Snake venoms and the hemostatic system. Toxicon. 1998;36:1749–800. - PubMed
-
- Koivunen E, Wang B, Ruoslahti E. Phage libraries displaying cyclic peptides with different ring sizes: ligand specificities of the RGD-directed integrins. Biotechnology (N Y) 1995;13:265–70. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 1R21CA135345/CA/NCI NIH HHS/United States
- U19 CA113298/CA/NCI NIH HHS/United States
- R01CA131015/CA/NCI NIH HHS/United States
- U19CA113298/CA/NCI NIH HHS/United States
- R01 CA115483/CA/NCI NIH HHS/United States
- P30 CA093373/CA/NCI NIH HHS/United States
- R03 CA129786/CA/NCI NIH HHS/United States
- R33 CA086364/CA/NCI NIH HHS/United States
- R33 CA099136/CA/NCI NIH HHS/United States
- R21 CA135345/CA/NCI NIH HHS/United States
- R01CA115483/CA/NCI NIH HHS/United States
- R33CA-99136/CA/NCI NIH HHS/United States
- R33CA-86364/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

