Electrochemical and homogeneous electron transfers to the Alzheimer amyloid-beta copper complex follow a preorganization mechanism
- PMID: 20858730
- PMCID: PMC2951405
- DOI: 10.1073/pnas.1011315107
Electrochemical and homogeneous electron transfers to the Alzheimer amyloid-beta copper complex follow a preorganization mechanism
Abstract
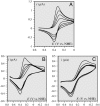
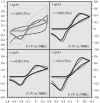
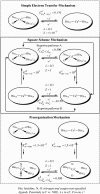
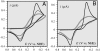
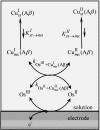
Deciphering the electron transfer reactivity characteristics of amyloid β-peptide copper complexes is an important task in connection with the role they are assumed to play in Alzheimer's disease. A systematic analysis of this question with the example of the amyloid β-peptide copper complex by means of its electrochemical current-potential responses and of its homogenous reactions with electrogenerated fast electron exchanging osmium complexes revealed a quite peculiar mechanism: The reaction proceeds through a small fraction of the complex molecules in which the peptide complex is "preorganized" so as the distances and angles in the coordination sphere to vary minimally upon electron transfer, thus involving a remarkably small reorganization energy (0.3 eV). This preorganization mechanism and its consequences on the reactivity should be taken into account for reactions involving dioxygen and hydrogen peroxide that are considered to be important in Alzheimer's disease through the production of harmful reactive oxygen species.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Redox reactions of copper complexes formed with different beta-amyloid peptides and their neuropathological [correction of neuropathalogical] relevance.Biochemistry. 2007 Aug 14;46(32):9270-82. doi: 10.1021/bi700508n. Epub 2007 Jul 18. Biochemistry. 2007. PMID: 17636872 Free PMC article.
-
Tyrosine gated electron transfer is key to the toxic mechanism of Alzheimer's disease beta-amyloid.FASEB J. 2004 Sep;18(12):1427-9. doi: 10.1096/fj.04-1890fje. Epub 2004 Jul 1. FASEB J. 2004. PMID: 15231727
-
Identifying, by first-principles simulations, Cu[amyloid-β] species making Fenton-type reactions in Alzheimer's disease.J Phys Chem B. 2013 Dec 27;117(51):16455-67. doi: 10.1021/jp410046w. Epub 2013 Dec 13. J Phys Chem B. 2013. PMID: 24313818
-
Electrochemistry of Alzheimer Disease Amyloid Beta Peptides.Curr Med Chem. 2018;25(33):4066-4083. doi: 10.2174/0929867325666180214112536. Curr Med Chem. 2018. PMID: 29446720 Review.
-
The amyloid beta peptide: a chemist's perspective. Role in Alzheimer's and fibrillization.Chem Rev. 2012 Oct 10;112(10):5147-92. doi: 10.1021/cr3000994. Epub 2012 Jul 19. Chem Rev. 2012. PMID: 22813427 Review. No abstract available.
Cited by
-
Redox processes in Cu-binding proteins: the "in-between" states in intrinsically disordered peptides.Chem Soc Rev. 2023 Oct 2;52(19):6595-6600. doi: 10.1039/d3cs00443k. Chem Soc Rev. 2023. PMID: 37701947 Free PMC article. Review.
-
An Electrochemistry and Computational Study at an Electrified Liquid-Liquid Interface for Studying Beta-Amyloid Aggregation.Membranes (Basel). 2023 Jun 5;13(6):584. doi: 10.3390/membranes13060584. Membranes (Basel). 2023. PMID: 37367788 Free PMC article.
-
Ascorbate Oxidation by Cu(Amyloid-β) Complexes: Determination of the Intrinsic Rate as a Function of Alterations in the Peptide Sequence Revealing Key Residues for Reactive Oxygen Species Production.Anal Chem. 2018 May 1;90(9):5909-5915. doi: 10.1021/acs.analchem.8b00740. Epub 2018 Apr 12. Anal Chem. 2018. PMID: 29611698 Free PMC article.
-
Metal Binding to Aβ Peptides Inhibits Interaction with Cytochrome c: Insights from Abiological Constructs.ACS Omega. 2018 Oct 25;3(10):13994-14003. doi: 10.1021/acsomega.8b01736. eCollection 2018 Oct 31. ACS Omega. 2018. PMID: 31458095 Free PMC article.
-
Role of PTA in the prevention of Cu(amyloid-β) induced ROS formation and amyloid-β oligomerisation in the presence of Zn.Metallomics. 2019 Jun 19;11(6):1154-1161. doi: 10.1039/c9mt00011a. Metallomics. 2019. PMID: 31098605 Free PMC article.
References
-
- Gaggelli E, Kozlowski H, Valensin D, Valensin G. Copper homeostasis and neurodegenerative disorders (Alzheimer’s, Prion, and Parkinson’s diseases and amyotrophic lateral sclerosis) Chem Rev. 2006;106:1995–2044. - PubMed
-
- Barnham KJ, Bush AI. Metals in Alzheimer’s and Parkinson’s diseases. Curr Opin Chem Biol. 2008;12:222–228. - PubMed
-
- Smith DG, Cappai R, Barnham KJ. The redox chemistry of the Alzheimer’s disease amyloid b peptide. Biochim Biophys Acta Biomembr. 2007;1768:1976–1990. - PubMed
-
- Hureau C, Faller P. Aβ-mediated ROS production by the Cu ions: Structural insights, mechanisms and relevance to Alzheimer’s disease. Biochimie. 2009;91:1212–1217. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

