Membrane-type MMPs are indispensable for placental labyrinth formation and development
- PMID: 20858856
- PMCID: PMC3031418
- DOI: 10.1182/blood-2009-10-249847
Membrane-type MMPs are indispensable for placental labyrinth formation and development
Abstract
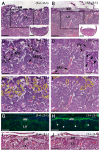
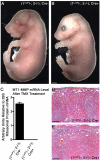
The membrane-type matrix metalloproteinases (MT-MMPs) are essential for pericellular matrix remodeling in late stages of development, as well as in growth and tissue homeostasis in postnatal life. Although early morphogenesis is perceived to involve substantial tissue remodeling, the roles of MT-MMPs in these processes are only partially characterized. Here we explore the functions of 2 prominently expressed MT-MMPs, MT1-MMP and MT2-MMP, and describe their roles in the process of placental morphogenesis. The fetal portion of the placenta, in particular the labyrinth (LA), displays strong overlapping expression of MT1-MMP and MT2-MMP, which is critical for syncytiotrophoblast formation and in turn for fetal vessels. Disruption of trophoblast syncytium formation consequently leads to developmental arrest with only a few poorly branched fetal vessels entering the LA causing embryonic death at embryonic day 11.5. Through knockdown of MMP expression, we demonstrate that either MT1-MMP or MT2-MMP is crucial specifically during development of the LA. In contrast, knockdown of MT-MMP activity after LA formation is compatible with development to term and postnatal life. Taken together these data identify essential but interchangeable roles for MT1-MMP or MT2-MMP in placental vasculogenesis and provide the first example of selective temporal and spatial MMP activity required for development of the mouse embryo.
Figures




Similar articles
-
MT2-MMP-dependent release of collagen IV NC1 domains regulates submandibular gland branching morphogenesis.Dev Cell. 2009 Oct;17(4):482-93. doi: 10.1016/j.devcel.2009.07.016. Dev Cell. 2009. PMID: 19853562 Free PMC article.
-
MT1-MMP expression in first-trimester placental tissue is upregulated in type 1 diabetes as a result of elevated insulin and tumor necrosis factor-alpha levels.Diabetes. 2008 Jan;57(1):150-7. doi: 10.2337/db07-0903. Epub 2007 Oct 10. Diabetes. 2008. PMID: 17928399
-
Expression of matrix metalloproteinase (MMP)-2, MMP-14 and tissue inhibitor of matrix metalloproteinase (TIMP)-2 during bovine placentation and at term with or without placental retention.Theriogenology. 2011 Apr 1;75(6):1104-14. doi: 10.1016/j.theriogenology.2010.11.019. Epub 2011 Jan 17. Theriogenology. 2011. PMID: 21247626
-
Placental membrane-type metalloproteinases (MT-MMPs): Key players in pregnancy.Cell Adh Migr. 2016 Mar 3;10(1-2):136-46. doi: 10.1080/19336918.2015.1110671. Epub 2016 Jan 8. Cell Adh Migr. 2016. PMID: 26745344 Free PMC article. Review.
-
Matrix invasion by tumour cells: a focus on MT1-MMP trafficking to invadopodia.J Cell Sci. 2009 Sep 1;122(Pt 17):3015-24. doi: 10.1242/jcs.034561. J Cell Sci. 2009. PMID: 19692588 Review.
Cited by
-
Functional roles of MMP14 and MMP15 in early postnatal mammary gland development.Development. 2016 Nov 1;143(21):3956-3968. doi: 10.1242/dev.136259. Epub 2016 Sep 15. Development. 2016. PMID: 27633994 Free PMC article.
-
Biochemical and Biological Attributes of Matrix Metalloproteinases.Prog Mol Biol Transl Sci. 2017;147:1-73. doi: 10.1016/bs.pmbts.2017.02.005. Epub 2017 Mar 22. Prog Mol Biol Transl Sci. 2017. PMID: 28413025 Free PMC article. Review.
-
Basement membrane perforations guide anterior-posterior axis formation.Nat Commun. 2025 Jul 22;16(1):6763. doi: 10.1038/s41467-025-61441-6. Nat Commun. 2025. PMID: 40695803 Free PMC article.
-
Mouse models of MYH9-related disease: mutations in nonmuscle myosin II-A.Blood. 2012 Jan 5;119(1):238-50. doi: 10.1182/blood-2011-06-358853. Epub 2011 Sep 8. Blood. 2012. PMID: 21908426 Free PMC article.
-
Interleukin-10: a novel metabolic inducer of macrophage differentiation and subsequently contributing to improved pregnancy outcomes of mice by orchestrating oxidative phosphorylation metabolism†.Biol Reprod. 2024 Jul 12;111(1):76-91. doi: 10.1093/biolre/ioae041. Biol Reprod. 2024. PMID: 38501817 Free PMC article.
References
-
- Abrahamsohn PA, Zorn TM. Implantation and decidualization in rodents. J Exp Zool. 1993;266(6):603–628. - PubMed
-
- Cross JC, Nakano H, Natale DR, Simmons DG, Watson ED. Branching morphogenesis during development of placental villi. Differentiation. 2006;74(7):393–401. - PubMed
-
- Rinkenberger JL, Cross JC, Werb Z. Molecular genetics of implantation in the mouse. Dev Genet. 1997;21(1):6–20. - PubMed
-
- Salamonsen LA, Dimitriadis E, Jones RL, Nie G. Complex regulation of decidualization: a role for cytokines and proteases–a review. Placenta. 2003;24(Suppl A):S76–S85. - PubMed
-
- Wang H, Dey SK. Roadmap to embryo implantation: clues from mouse models. Nat Rev Genet. 2006;7(3):185–99. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

