Ca2+ overload and sarcoplasmic reticulum instability in tric-a null skeletal muscle
- PMID: 20858894
- PMCID: PMC2988342
- DOI: 10.1074/jbc.M110.170084
Ca2+ overload and sarcoplasmic reticulum instability in tric-a null skeletal muscle
Abstract
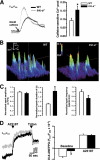
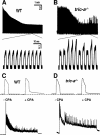
The sarcoplasmic reticulum (SR) of skeletal muscle contains K(+), Cl(-), and H(+) channels may facilitate charge neutralization during Ca(2+) release. Our recent studies have identified trimeric intracellular cation (TRIC) channels on SR as an essential counter-ion permeability pathway associated with rapid Ca(2+) release from intracellular stores. Skeletal muscle contains TRIC-A and TRIC-B isoforms as predominant and minor components, respectively. Here we test the physiological function of TRIC-A in skeletal muscle. Biochemical assay revealed abundant expression of TRIC-A relative to the skeletal muscle ryanodine receptor with a molar ratio of TRIC-A/ryanodine receptor ∼5:1. Electron microscopy with the tric-a(-/-) skeletal muscle showed Ca(2+) overload inside the SR with frequent formation of Ca(2+) deposits compared with the wild type muscle. This elevated SR Ca(2+) pool in the tric-a(-/-) muscle could be released by caffeine, whereas the elemental Ca(2+) release events, e.g. osmotic stress-induced Ca(2+) spark activities, were significantly reduced likely reflecting compromised counter-ion movement across the SR. Ex vivo physiological test identified the appearance of "alternan" behavior with isolated tric-a(-/-) skeletal muscle, i.e. transient and drastic increase in contractile force appeared within the decreasing force profile during repetitive fatigue stimulation. Inhibition of SR/endoplasmic reticulum Ca(2+ ATPase) function could lead to aggravation of the stress-induced alternans in the tric-a(-/-) muscle. Our data suggests that absence of TRIC-A may lead to Ca(2+) overload in SR, which in combination with the reduced counter-ion movement may lead to instability of Ca(2+) movement across the SR membrane. The observed alternan behavior with the tric-a(-/-) muscle may reflect a skeletal muscle version of store overload-induced Ca(2+) release that has been reported in the cardiac muscle under stress conditions.
Figures


References
-
- Ríos E., Ma J. J., González A. (1991) J. Muscle Res. Cell Motil. 12, 127–135 - PubMed
-
- Melzer W., Herrmann-Frank A., Lüttgau H. C. (1995) Biochim. Biophys. Acta 1241, 59–116 - PubMed
-
- Fill M., Copello J. A. (2002) Physiol. Rev. 82, 893–922 - PubMed
-
- Takeshima H., Komazaki S., Nishi M., Iino M., Kangawa K. (2000) Mol. Cell 6, 11–22 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

