Cone and rod photoreceptor transplantation in models of the childhood retinopathy Leber congenital amaurosis using flow-sorted Crx-positive donor cells
- PMID: 20858907
- PMCID: PMC2972691
- DOI: 10.1093/hmg/ddq378
Cone and rod photoreceptor transplantation in models of the childhood retinopathy Leber congenital amaurosis using flow-sorted Crx-positive donor cells
Abstract
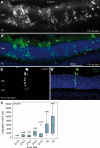
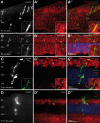
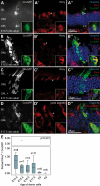
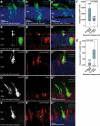
Retinal degenerative disease causing loss of photoreceptor cells is the leading cause of untreatable blindness in the developed world, with inherited degeneration affecting 1 in 3000 people. Visual acuity deteriorates rapidly once the cone photoreceptors die, as these cells provide daylight and colour vision. Here, in proof-of-principle experiments, we demonstrate the feasibility of cone photoreceptor transplantation into the wild-type and degenerating retina of two genetic models of Leber congenital amaurosis, the Crb1(rd8/rd8) and Gucy2e(-/-) mouse. Crx-expressing cells were flow-sorted from the developing retina of CrxGFP transgenic mice and transplanted into adult recipient retinae; CrxGFP is a marker of cone and rod photoreceptor commitment. Only the embryonic-stage Crx-positive donor cells integrated within the outer nuclear layer of the recipient and differentiated into new cones, whereas postnatal cells generated a 10-fold higher number of rods compared with embryonic-stage donors. New cone photoreceptors displayed unambiguous morphological cone features and expressed mature cone markers. Importantly, we found that the adult environment influences the number of integrating cones and favours rod integration. New cones and rods were observed in ratios similar to that of the host retina (1:35) even when the transplanted population consisted primarily of cone precursors. Cone integration efficiency was highest in the cone-deficient Gucy2e(-/-) retina suggesting that cone depletion creates a more optimal environment for cone transplantation. This is the first comprehensive study demonstrating the feasibility of cone transplantation into the adult retina. We conclude that flow-sorted embryonic-stage Crx-positive donor cells have the potential to replace lost cones, as well as rods, an important requirement for retinal disease therapy.
Figures


Similar articles
-
Cone-rod homeobox CRX controls presynaptic active zone formation in photoreceptors of mammalian retina.Hum Mol Genet. 2018 Oct 15;27(20):3555-3567. doi: 10.1093/hmg/ddy272. Hum Mol Genet. 2018. PMID: 30084954 Free PMC article.
-
CRX Expression in Pluripotent Stem Cell-Derived Photoreceptors Marks a Transplantable Subpopulation of Early Cones.Stem Cells. 2019 May;37(5):609-622. doi: 10.1002/stem.2974. Epub 2019 Jan 30. Stem Cells. 2019. PMID: 30681766 Free PMC article.
-
Daylight vision repair by cell transplantation.Stem Cells. 2015 Jan;33(1):79-90. doi: 10.1002/stem.1824. Stem Cells. 2015. PMID: 25183393
-
Cone Health and Retinoids.Prog Mol Biol Transl Sci. 2015;134:465-76. doi: 10.1016/bs.pmbts.2015.06.002. Epub 2015 Jul 2. Prog Mol Biol Transl Sci. 2015. PMID: 26310171 Review.
-
An overview of Leber congenital amaurosis: a model to understand human retinal development.Surv Ophthalmol. 2004 Jul-Aug;49(4):379-98. doi: 10.1016/j.survophthal.2004.04.003. Surv Ophthalmol. 2004. PMID: 15231395 Review.
Cited by
-
The Prospects for Retinal Organoids in Treatment of Retinal Diseases.Asia Pac J Ophthalmol (Phila). 2022 Jul-Aug 01;11(4):314-327. doi: 10.1097/APO.0000000000000538. Epub 2022 Aug 30. Asia Pac J Ophthalmol (Phila). 2022. PMID: 36041146 Free PMC article. Review.
-
Isolation of Human Photoreceptor Precursors via a Cell Surface Marker Panel from Stem Cell-Derived Retinal Organoids and Fetal Retinae.Stem Cells. 2018 May;36(5):709-722. doi: 10.1002/stem.2775. Epub 2018 Feb 1. Stem Cells. 2018. PMID: 29327488 Free PMC article.
-
Material Exchange in Photoreceptor Transplantation: Updating Our Understanding of Donor/Host Communication and the Future of Cell Engraftment Science.Front Neural Circuits. 2018 Mar 6;12:17. doi: 10.3389/fncir.2018.00017. eCollection 2018. Front Neural Circuits. 2018. PMID: 29559897 Free PMC article. Review.
-
Outer retinal tubulations in chronic central serous chorioretinopathy.Graefes Arch Clin Exp Ophthalmol. 2013 Jun;251(6):1655-6. doi: 10.1007/s00417-012-2151-0. Epub 2012 Sep 7. Graefes Arch Clin Exp Ophthalmol. 2013. PMID: 22955718 No abstract available.
-
Short-Term Omega-3 Supplementation Modulates Novel Neurovascular and Fatty Acid Metabolic Proteome Changes in the Retina and Ophthalmic Artery of Mice with Targeted Cyp2c44 Gene Deletion.Cells. 2022 Nov 4;11(21):3494. doi: 10.3390/cells11213494. Cells. 2022. PMID: 36359890 Free PMC article.
References
-
- Morrow E.M., Furukawa T., Cepko C.L. Vertebrate photoreceptor cell development and disease. Trends Cell Biol. 1998;8:353–358. doi:10.1016/S0962-8924(98)01341-5. - DOI - PubMed
-
- Hartong D.T., Berson E.L., Dryja T.P. Retinitis pigmentosa. Lancet. 2006;368:1795–1809. doi:10.1016/S0140-6736(06)69740-7. - DOI - PubMed
-
- Lamba D.A., Karl M.O., Reh T.A. Strategies for retinal repair: cell replacement and regeneration. Prog. Brain Res. 2009;175:23–31. doi:10.1016/S0079-6123(09)17502-7. - DOI - PubMed
-
- West E.L., Pearson R.A., MacLaren R.E., Sowden J.C., Ali R.R. Cell transplantation strategies for retinal repair. Prog. Brain Res. 2009;175:3–21. doi:10.1016/S0079-6123(09)17501-5. - DOI - PMC - PubMed
-
- MacLaren R.E., Pearson R.A., MacNeil A., Douglas R.H., Salt T.E., Akimoto M., Swaroop A., Sowden J.C., Ali R.R. Retinal repair by transplantation of photoreceptor precursors. Nature. 2006;444:203–207. doi:10.1038/nature05161. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

