Negative BOLD-fMRI signals in large cerebral veins
- PMID: 20859295
- PMCID: PMC3049531
- DOI: 10.1038/jcbfm.2010.164
Negative BOLD-fMRI signals in large cerebral veins
Abstract
Reductions in blood oxygenation level dependent (BOLD)-functional magnetic resonance imaging (fMRI) signals below baseline levels have been observed under several conditions as negative activation in task-activation studies or anticorrelation in resting-state experiments. Converging evidence suggests that negative BOLD signals (NBSs) can generally be explained by local reductions in neural activity. Here, we report on NBSs that accompany hemodynamic changes in regions devoid of neural tissue. The NBSs were investigated with high-resolution studies of the visual cortex (VC) at 7 T. Task-activation studies were performed to localize a task-positive area in the VC. During rest, robust negative correlation with the task-positive region was observed in focal regions near the ventricles and dispersed throughout the VC. Both positive and NBSs were dependent on behavioral condition. Comparison with high-resolution structural images showed that negatively correlated regions overlapped with larger pial and ependymal veins near sulcal and ventricular cerebrospinal fluid (CSF). Results from multiecho fMRI showed that NBSs were consistent with increases in local blood volume. These findings confirm theoretical predictions that tie neural activity to blood volume increases, which tend to counteract positive fMRI signal changes associated with increased blood oxygenation. This effect may be more salient in high-resolution studies, in which positive and NBS may be more often spatially distinct.
Figures

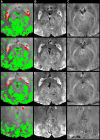
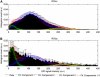
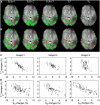
References
-
- Allison JD, Meador KJ, Loring DW, Figueroa RE, Wright JC. Functional MRI cerebral activation and deactivation during finger movement. Neurology. 2000;54:135–142. - PubMed
-
- Birn RM, Diamond JB, Smith MA, Bandettini PA. Separating respiratory-variation-related fluctuations from neuronal-activity-related fluctuations in fMRI. Neuroimage. 2006;31:1536–1548. - PubMed
-
- Blockley NP, Jiang L, Gardener AG, Ludman CN, Francis ST, Gowland PA. Field strength dependence of R1 and R2* relaxivities of human whole blood to ProHance, Vasovist, and deoxyhemoglobin. Magn Reson Med. 2008;60:1313–1320. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

