Involvement of M2-polarized macrophages in the ascites from advanced epithelial ovarian carcinoma in tumor progression via Stat3 activation
- PMID: 20860602
- PMCID: PMC11159803
- DOI: 10.1111/j.1349-7006.2010.01652.x
Involvement of M2-polarized macrophages in the ascites from advanced epithelial ovarian carcinoma in tumor progression via Stat3 activation
Abstract
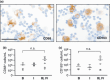
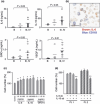
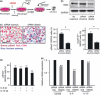
Ascites macrophages in advanced epithelial ovarian cancer (AdEOC) are involved in cancer metastasis and progression by modifying the tumor microenvironment. However, the precise mechanisms of cell-to-cell interaction between macrophages and tumor cells are still unclear. This study focused on the activation of signal transducer and activator of transcription 3 (Stat3) which is a critical signal transduction molecule at a point of convergence for numerous oncogenic signaling pathways as well as controlling the M2-poralization of macrophages. AdEOC ascites, in which high concentration of interleukin (IL)-6, IL-10, growth-related oncogene-alpha and vascular endothelial growth factor were detected, stimulated the proliferation of SKOV3 cells, a human ovarian cancer cell line. The simultaneous blocking of IL-6 and IL-10 by neutralizing antibodies suppressed ascites-induced tumor cell proliferation. Stat3 activation in SKOV3 cells was induced by co-culture with macrophages especially with macrophage colony stimulating factor-primed M2 macrophages but lesser extent with granulocyte-macrophage colony stimulating factor-primed immature macrophages. Cyclin-D1 expression in SKOV3 cells was also significantly induced by co-culture with macrophages. Blocking of Stat3 in macrophages by small interfering RNA inhibited the production of IL-6 and IL-10 by macrophages, and suppressed Stat3 activation and cyclin-D1 induction in co-cultured SKOV3 cells. Stat3 activation in SKOV3 cells was abrogated by simultaneous neutralization of IL-6 and IL-10. These results indicate that Stat3 activation by IL-6 and IL-10 plays an important role in cell-to-cell interaction between tumor cells and macrophages in the ascites of AdEOC.
© 2010 Japanese Cancer Association.
Figures




References
-
- Okamura H, Katabuchi H. Pathophysiological dynamics of human ovarian surface epithelial cells in epithelial ovarian carcinogenesis. Int Rev Cytol 2005; 242: 1–54. - PubMed
-
- Bhoola S, Hoskins WJ. Diagnosis and management of epithelial ovarian cancer. Obstet Gynecol 2006; 107: 1399–1410. - PubMed
-
- Shield K, Ackland ML, Ahmed N, Rice GE. Multicellular spheroids in ovarian cancer metastases: biology and pathology. Gynecol Oncol 2009; 113: 143–148. - PubMed
-
- Okamura H, Katabuchi H, Kanzaki H. Macrophages in reproductive biology. In: Lewis C, Burke B, eds. The Macrophages. London: Oxford University Press, 2002; 548–576.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

