Optimization of the degenerated interfacial ATP binding site improves the function of disease-related mutant cystic fibrosis transmembrane conductance regulator (CFTR) channels
- PMID: 20861014
- PMCID: PMC2988371
- DOI: 10.1074/jbc.M110.172817
Optimization of the degenerated interfacial ATP binding site improves the function of disease-related mutant cystic fibrosis transmembrane conductance regulator (CFTR) channels
Abstract
The cystic fibrosis transmembrane conductance regulator (CFTR) chloride channel, an ATP binding cassette (ABC) protein whose defects cause the deadly genetic disease cystic fibrosis (CF), encompasses two nucleotide binding domains (NBD1 and NBD2). Recent studies indicate that in the presence of ATP, the two NBDs coalesce into a dimer, trapping an ATP molecule in each of the two interfacial composite ATP binding sites (site 1 and site 2). Experimental evidence also suggests that CFTR gating is mainly controlled by ATP binding and hydrolysis in site 2, whereas site 1, which harbors several non-canonical substitutions in ATP-interacting motifs, is considered degenerated. The CF-associated mutation G551D, by introducing a bulky and negatively charged side chain into site 2, completely abolishes ATP-induced openings of CFTR. Here, we report a strategy to optimize site 1 for ATP binding by converting two amino acid residues to ABC consensus (i.e. H1348G) or more commonly seen residues in other ABC proteins (i.e. W401Y,W401F). Introducing either one or both of these mutations into G551D-CFTR confers ATP responsiveness for this disease-associated mutant channel. We further showed that the same maneuver also improved the function of WT-CFTR and the most common CF-associated ΔF508 channels, both of which rely on site 2 for gating control. Thus, our results demonstrated that the degenerated site 1 can be rebuilt to complement or support site 2 for CFTR function. Possible approaches for developing CFTR potentiators targeting site 1 will be discussed.
Figures
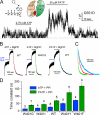
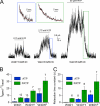
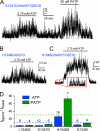

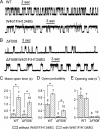
References
-
- Davidson A. L., Chen J. (2004) Annu. Rev. Biochem. 73, 241–268 - PubMed
-
- Oswald C., Holland I. B., Schmitt L. (2006) Naunyn Schmiedebergs Arch. Pharmacol. 372, 385–399 - PubMed
-
- Procko E., O'Mara M. L., Bennett W. F., Tieleman D. P., Gaudet R. (2009) FASEB J. 23, 1287–1302 - PubMed
-
- Riordan J. R., Rommens J. M., Kerem B., Alon N., Rozmahel R., Grzelczak Z., Zielenski J., Lok S., Plavsic N., Chou J. L., et al. (1989) Science 245, 1066–1073 - PubMed
-
- Chen T. Y., Hwang T. C. (2008) Physiol. Rev. 88, 351–387 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

