AMP-activated protein kinase inhibits KCNQ1 channels through regulation of the ubiquitin ligase Nedd4-2 in renal epithelial cells
- PMID: 20861072
- PMCID: PMC3006313
- DOI: 10.1152/ajprenal.00423.2010
AMP-activated protein kinase inhibits KCNQ1 channels through regulation of the ubiquitin ligase Nedd4-2 in renal epithelial cells
Abstract
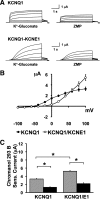
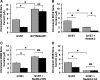
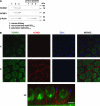
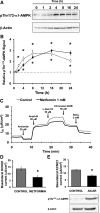
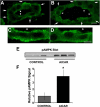
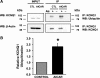
The KCNQ1 K(+) channel plays a key role in the regulation of several physiological functions, including cardiac excitability, cardiovascular tone, and body electrolyte homeostasis. The metabolic sensor AMP-activated protein kinase (AMPK) has been shown to regulate a growing number of ion transport proteins. To determine whether AMPK regulates KCNQ1, we studied the effects of AMPK activation on KCNQ1 currents in Xenopus laevis oocytes and collecting duct epithelial cells. AMPK activation decreased KCNQ1 currents and channel surface expression in X. laevis oocytes, but AMPK did not phosphorylate KCNQ1 in vitro, suggesting an indirect regulatory mechanism. As it has been recently shown that the ubiquitin-protein ligase Nedd4-2 inhibits KCNQ1 plasma membrane expression and that AMPK regulates epithelial Na(+) channels via Nedd4-2, we examined the role of Nedd4-2 in the AMPK-dependent regulation of KCNQ1. Channel inhibition by AMPK was blocked in oocytes coexpressing either a dominant-negative or constitutively active Nedd4-2 mutant, or a Nedd4-2 interaction-deficient KCNQ1 mutant, suggesting that Nedd4-2 participates in the regulation of KCNQ1 by AMPK. KCNQ1 is expressed at the basolateral membrane in mouse polarized kidney cortical collecting duct (mpkCCD(c14)) cells and in rat kidney. Treatment with the AMPK activators AICAR (2 mM) or metformin (1 mM) reduced basolateral KCNQ1 currents in apically permeabilized polarized mpkCCD(c14) cells. Moreover, AICAR treatment of rat kidney slices ex vivo induced AMPK activation and intracellular redistribution of KCNQ1 from the basolateral membrane in collecting duct principal cells. AICAR treatment also induced increased ubiquitination of KCNQ1 immunoprecipitated from kidney slice homogenates. These results indicate that AMPK inhibits KCNQ1 activity by promoting Nedd4-2-dependent channel ubiquitination and retrieval from the plasma membrane.
Figures


References
-
- Abriel H, Staub O. Ubiquitylation of ion channels. Physiology (Bethesda) 20: 398–407, 2005 - PubMed
-
- Acharya P, Beckel J, Ruiz WG, Wang E, Rojas R, Birder L, Apodaca G. Distribution of the tight junction proteins ZO-1, occludin, and claudin-4, -8, and -12 in bladder epithelium. Am J Physiol Renal Physiol 287: F305–F318, 2004 - PubMed
-
- Bacallao R, Stelzer EH. Preservation of biological specimens for observation in a confocal fluorescence microscope and operational principles of confocal fluorescence microscopy. Methods Cell Biol 31: 437–452, 1989 - PubMed
-
- Belfodil R, Barriere H, Rubera I, Tauc M, Poujeol C, Bidet M, Poujeol P. CFTR-dependent and -independent swelling-activated K+ currents in primary cultures of mouse nephron. Am J Physiol Renal Physiol 284: F812–F828, 2003 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

