PPARgamma-dependent regulation of adenylate cyclase 6 amplifies the stimulatory effect of cAMP on renin gene expression
- PMID: 20861226
- PMCID: PMC5417377
- DOI: 10.1210/me.2010-0134
PPARgamma-dependent regulation of adenylate cyclase 6 amplifies the stimulatory effect of cAMP on renin gene expression
Abstract
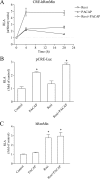
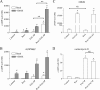
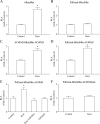
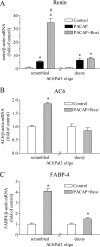
The second messenger cAMP plays an important role in the regulation of renin gene expression. Nuclear receptor peroxisome proliferator-activated receptor-γ (PPARγ) is known to stimulate renin gene transcription acting through PPARγ-binding sequences in renin promoter. We show now that activation of PPARγ by unsaturated fatty acids or thiazolidinediones drastically augments the cAMP-dependent increase of renin mRNA in the human renin-producing cell line Calu-6. The underlying mechanism involves potentiation of agonist-induced cAMP increase and up-regulation of adenylate cyclase 6 (AC6) gene expression. We identified a palindromic element with a 3-bp spacer (Pal3) in AC6 intron 1 (AC6Pal3). AC6Pal3 bound PPARγ and mediated trans-activation by PPARγ agonist. AC6 knockdown decreased basal renin mRNA level and attenuated the maximal PPARγ-dependent stimulation of the cAMP-induced renin gene expression. AC6Pal3 decoy oligonucleotide abrogated the PPARγ-dependent potentiation of cAMP-induced renin gene expression. Treatment of mice with PPARγ agonist increased AC6 mRNA kidney levels. Our data suggest that in addition to its direct effect on renin gene transcription, PPARγ "sensitizes" renin gene to cAMP via trans-activation of AC6 gene. AC6 has been identified as PPARγ target gene with a functional Pal3 sequence.
Figures





References
-
- Castrop H, Höcherl K, Kurtz A, Schweda F, Todorov V, Wagner C2010. Physiology of kidney renin. Physiol Rev 90:607–673 - PubMed
-
- Pan L, Gross KW2005. Transcriptional regulation of renin: an update. Hypertension 45:3–8 - PubMed
-
- Todorov VT, Desch M, Schmitt-Nilson N, Todorova A, Kurtz A2007. Peroxisome proliferator-activated receptor-γ is involved in the control of renin gene expression. Hypertension 50:939–944 - PubMed
-
- Todorov VT, Desch M, Schubert T, Kurtz A2008. The Pal3 promoter sequence is critical for the regulation of human renin gene transcription by peroxisome proliferator-activated receptor-γ. Endocrinology 149:4647–4657 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

