Cascade of events governing cell-cell fusion induced by herpes simplex virus glycoproteins gD, gH/gL, and gB
- PMID: 20861251
- PMCID: PMC2976417
- DOI: 10.1128/JVI.01700-10
Cascade of events governing cell-cell fusion induced by herpes simplex virus glycoproteins gD, gH/gL, and gB
Abstract
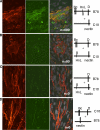
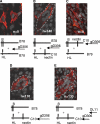
Herpesviruses minimally require the envelope proteins gB and gH/gL for virus entry and cell-cell fusion; herpes simplex virus (HSV) additionally requires the receptor-binding protein gD. Although gB is a class III fusion protein, gH/gL does not resemble any documented viral fusion protein at a structural level. Based on those data, we proposed that gH/gL does not function as a cofusogen with gB but instead regulates the fusogenic activity of gB. Here, we present data to support that hypothesis. First, receptor-positive B78H1-C10 cells expressing gH/gL fused with receptor-negative B78H1 cells expressing gB and gD (fusion in trans). Second, fusion occurred when gH/gL-expressing C10 cells preexposed to soluble gD were subsequently cocultured with gB-expressing B78 cells. In contrast, prior exposure of gB-expressing C10 cells to soluble gD did not promote subsequent fusion with gH/gL-expressing B78 cells. These data suggest that fusion involves activation of gH/gL by receptor-bound gD. Most importantly, soluble gH/gL triggered a low level of fusion of C10 cells expressing gD and gB; a much higher level was achieved when gB-expressing C10 cells were exposed to a combination of soluble gH/gL and gD. These data clearly show that gB acts as the HSV fusogen following activation by gD and gH/gL. We suggest the following steps leading to fusion: (i) conformational changes to gD upon receptor binding, (ii) alteration of gH/gL by receptor-activated gD, and (iii) upregulation of the fusogenic potential of gB following its interaction with activated gH/gL. The third step may be common to other herpesviruses.
Figures



References
-
- Atanasiu, D., J. C. Whitbeck, M. P. de Leon, H. Lou, B. P. Hannah, G. H. Cohen, and R. J. Eisenberg. 2010. Bimolecular complementation defines functional regions of herpes simplex virus gB that are involved with gH/gL as a necessary step leading to cell fusion. J. Virol. 84:3825-3834. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

