MAL/VIP17, a new player in the regulation of NKCC2 in the kidney
- PMID: 20861303
- PMCID: PMC2982131
- DOI: 10.1091/mbc.E10-05-0456
MAL/VIP17, a new player in the regulation of NKCC2 in the kidney
Abstract
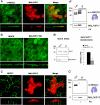
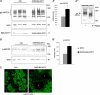
The renal-specific Na+-K+-2Cl- cotransporter (NKCC2) is the major salt transport pathway of the apical membrane of the mammalian thick ascending limb of Henle's loop. Here, we analyze the role of the tetraspan protein myelin and lymphocytes-associated protein (MAL)/VIP17 in the regulation of NKCC2. We demonstrated that 1) NKCC2 and MAL/VIP17 colocalize and coimmunoprecipitate in Lilly Laboratories cell porcine kidney cells (LLC-PK1) as well as in rat kidney medullae, 2) a 150-amino acid stretch of NKCC2 C-terminal tail is involved in the interaction with MAL/VIP17, 3) MAL/VIP17 increases the cell surface retention of NKCC2 by attenuating its internalization, and 4) this coincides with an increase in cotransporter phosphorylation. Interestingly, overexpression of MAL/VIP17 in the kidney of transgenic mice results in cysts formation in distal nephron structures consistent with the hypothesis that MAL/VIP17 plays an important role in apical sorting or in maintaining the stability of the apical membrane. The NKCC2 expressed in these mice was highly glycosylated and phosphorylated, suggesting that MAL/VIP17 also is involved in the stabilization of NKCC2 at the apical membrane in vivo. Thus, the involvement of MAL/VIP17 in the activation and surface expression of NKCC2 could play an important role in the regulated absorption of Na+ and Cl- in the kidney.
Figures

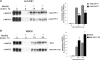



References
-
- Alvarez-Guerra M., Garay R. P. Renal Na-K-Cl cotransporter NKCC2 in Dahl salt-sensitive rats. J. Hypertension. 2002;20:721–727. - PubMed
-
- Caplan M. J., Kamsteeg E. J., Duffield A. Tetraspan proteins: regulators of renal structure and function. Curr. Opin. Nephrol. Hypertens. 2007;16:353–358. - PubMed
-
- Charrin S., le Naour F., Silvie O., Milhiet P. E., Boucheix C., Rubinstein E. Lateral organization of membrane proteins: tetraspanins spin their web. Biochem. J. 2009;420:133–154. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

