Hypoxia suppression of Bim and Bmf blocks anoikis and luminal clearing during mammary morphogenesis
- PMID: 20861305
- PMCID: PMC2982135
- DOI: 10.1091/mbc.E10-04-0353
Hypoxia suppression of Bim and Bmf blocks anoikis and luminal clearing during mammary morphogenesis
Abstract
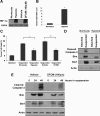
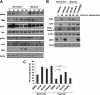
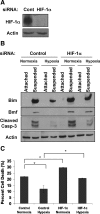
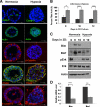
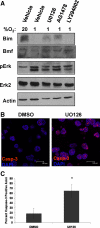
Proper adhesion to extracellular matrix is critical for epithelial cell survival. Detachment from matrix signals results in apoptosis, referred to as anoikis. Selective apoptosis of cells that become detached from matrix is associated with the formation of a lumen in three-dimensional mammary epithelial acinar structures in vitro. Because early breast cancer lesions such as carcinoma in situ, characterized by ducts exhibiting lumens filled with cells, are often associated with hypoxic markers, we sought to examine the role of hypoxia in anoikis and lumen formation in mammary epithelial cells. Here, we show that hypoxic conditions inhibit anoikis and block expression of proapoptotic BH3-only family members Bim and Bmf in epithelial cells. Hypoxia-mediated anoikis protection is associated with increased activation of the epidermal growth factor receptor-mitogen-activated protein kinase kinase-extracellular signal-regulated kinase (Erk) kinase pathway and requires the hypoxia-activated transcription factor. Consistent with these data, hypoxic conditions inhibit luminal clearing during morphogenesis in human mammary epithelial acini when grown in three-dimensional cultures and are associated with decreased expression of Bim and Bmf as well as Erk activation. We show that hypoxia regulates specific cell survival pathways that disrupt tissue architecture related to clearing of luminal space during mammary morphogenesis and suggest that hypoxia-mediated anoikis resistance may contribute to cancer progression.
Figures
Similar articles
-
Surviving without oxygen: hypoxia regulation of mammary morphogenesis and anoikis.Cell Cycle. 2011 Jul 15;10(14):2287-94. doi: 10.4161/cc.10.14.16532. Epub 2011 Jul 15. Cell Cycle. 2011. PMID: 21670595
-
Bim regulation of lumen formation in cultured mammary epithelial acini is targeted by oncogenes.Mol Cell Biol. 2005 Jun;25(11):4591-601. doi: 10.1128/MCB.25.11.4591-4601.2005. Mol Cell Biol. 2005. PMID: 15899862 Free PMC article.
-
ErbB2 requires integrin alpha5 for anoikis resistance via Src regulation of receptor activity in human mammary epithelial cells.J Cell Sci. 2010 Apr 15;123(Pt 8):1373-82. doi: 10.1242/jcs.050906. Epub 2010 Mar 23. J Cell Sci. 2010. PMID: 20332114 Free PMC article.
-
Lumen formation during mammary epithelial morphogenesis: insights from in vitro and in vivo models.Cell Cycle. 2008 Jan 1;7(1):57-62. doi: 10.4161/cc.7.1.5150. Epub 2007 Oct 9. Cell Cycle. 2008. PMID: 18196964 Review.
-
Metabolic Stress Adaptations Underlie Mammary Gland Morphogenesis and Breast Cancer Progression.Cells. 2021 Oct 2;10(10):2641. doi: 10.3390/cells10102641. Cells. 2021. PMID: 34685621 Free PMC article. Review.
Cited by
-
The role of multicellular aggregation in the survival of ErbB2-positive breast cancer cells during extracellular matrix detachment.J Biol Chem. 2015 Apr 3;290(14):8722-33. doi: 10.1074/jbc.M114.612754. Epub 2015 Feb 13. J Biol Chem. 2015. PMID: 25681438 Free PMC article.
-
Timeless preserves telomere length by promoting efficient DNA replication through human telomeres.Cell Cycle. 2012 Jun 15;11(12):2337-47. doi: 10.4161/cc.20810. Epub 2012 Jun 15. Cell Cycle. 2012. PMID: 22672906 Free PMC article.
-
The oncogene HER2/neu (ERBB2) requires the hypoxia-inducible factor HIF-1 for mammary tumor growth and anoikis resistance.J Biol Chem. 2013 May 31;288(22):15865-77. doi: 10.1074/jbc.M112.426999. Epub 2013 Apr 12. J Biol Chem. 2013. PMID: 23585570 Free PMC article.
-
Oxygen and metabolic reprogramming in the tumor microenvironment influences metastasis homing.Cancer Biol Ther. 2021 Dec 2;22(10-12):493-512. doi: 10.1080/15384047.2021.1992233. Epub 2021 Oct 25. Cancer Biol Ther. 2021. PMID: 34696706 Free PMC article. Review.
-
Pokemon inhibits Bim transcription to promote the proliferation, anti-anoikis, invasion, histological grade, and dukes stage of colorectal neoplasms.J Cancer Res Clin Oncol. 2024 Aug 3;150(8):380. doi: 10.1007/s00432-024-05904-1. J Cancer Res Clin Oncol. 2024. PMID: 39095579 Free PMC article.
References
-
- Bedogni B., Welford S. M., Cassarino D. S., Nickoloff B. J., Giaccia A. J., Powell M. B. The hypoxic microenvironment of the skin contributes to Akt-mediated melanocyte transformation. Cancer Cell. 2005;8:443–454. - PubMed
-
- Belloc F., Moreau-Gaudry F., Uhalde M., Cazalis L., Jeanneteau M., Lacombe F., Praloran V., Mahon F. X. Imatinib and nilotinib induce apoptosis of chronic myeloid leukemia cells through a Bim-dependant pathway modulated by cytokines. Cancer Biol. Ther. 2007;6:912–919. - PubMed
-
- Bos R., Zhong H., Hanrahan C. F., Mommers E. C., Semenza G. L., Pinedo H. M., Abeloff M. D., Simons J. W., van Diest P. J., van der Wall E., et al. Levels of hypoxia-inducible factor-1 alpha during breast carcinogenesis. J. Natl. Cancer Inst. 2001;93:309–314. - PubMed
-
- Cabodi S., Moro L., Bergatto E., Boeri Erba E., Di Stefano P., Turco E., Tarone G., Defilippi P. Integrin regulation of epidermal growth factor (EGF) receptor and of EGF-dependent responses. Biochem. Soc. Trans. 2004;32:438–442. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

