Cutting edge: NLRP12 controls dendritic and myeloid cell migration to affect contact hypersensitivity
- PMID: 20861349
- PMCID: PMC3641837
- DOI: 10.4049/jimmunol.1002227
Cutting edge: NLRP12 controls dendritic and myeloid cell migration to affect contact hypersensitivity
Abstract
Nucleotide-binding domain leucine-rich repeat (NLR) proteins are regulators of inflammation and immunity. Although first described 8 y ago, a physiologic role for NLRP12 has remained elusive until now. We find that murine Nlrp12, an NLR linked to atopic dermatitis and hereditary periodic fever in humans, is prominently expressed in dendritic cells (DCs) and neutrophils. Nlrp12-deficient mice exhibit attenuated inflammatory responses in two models of contact hypersensitivity that exhibit features of allergic dermatitis. This cannot be attributed to defective Ag processing/presentation, inflammasome activation, or measurable changes in other inflammatory cytokines. Rather, Nlrp12(-/-) DCs display a significantly reduced capacity to migrate to draining lymph nodes. Both DCs and neutrophils fail to respond to chemokines in vitro. These findings indicate that NLRP12 is important in maintaining neutrophils and peripheral DCs in a migration-competent state.
Figures


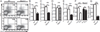

 ) BMDCs to the indicated chemokine. Data are representative of 3–5 experiments and are presented as mean ± SEM of one experiment. Data from all experiments with associated pairwise comparison statistics are presented in table S4. (I) Migration of WT and Nlrp12−/− neutrophils to CXCL1. Data are comprised of three independent experiments, presented as mean ± SEM, and pairwise comparisons were made using two-tailed Student’s t test, α = 0.05. * P<0.05.
) BMDCs to the indicated chemokine. Data are representative of 3–5 experiments and are presented as mean ± SEM of one experiment. Data from all experiments with associated pairwise comparison statistics are presented in table S4. (I) Migration of WT and Nlrp12−/− neutrophils to CXCL1. Data are comprised of three independent experiments, presented as mean ± SEM, and pairwise comparisons were made using two-tailed Student’s t test, α = 0.05. * P<0.05.References
-
- Kuenzel S, Till A, Winkler M, Hasler R, Lipinski S, Jung S, Grotzinger J, Fickenscher H, Schreiber S, Rosenstiel P. The nucleotide-binding oligomerization domain-like receptor NLRC5 is involved in IFN-dependent antiviral immune responses. J Immunol. 2010;184:1990–2000. - PubMed
-
- Lich JD, Williams KL, Moore CB, Arthur JC, Davis BK, Taxman DJ, Ting JP. Monarch-1 suppresses non-canonical NF-kappaB activation and p52-dependent chemokine expression in monocytes. J Immunol. 2007;178:1256–1260. - PubMed
-
- Williams KL, Lich JD, Duncan JA, Reed W, Rallabhandi P, Moore C, Kurtz S, Coffield VM, Accavitti-Loper MA, Su L, Vogel SN, Braunstein M, Ting JP. The CATERPILLER protein monarch-1 is an antagonist of toll-like receptor-, tumor necrosis factor alpha-, Mycobacterium tuberculosis-induced pro-inflammatory signals. J Biol Chem. 2005;280:39914–39924. - PMC - PubMed
-
- Jeru I, Duquesnoy P, Fernandes-Alnemri T, Cochet E, Yu JW, Lackmy-Port-Lis M, Grimprel E, Landman-Parker J, Hentgen V, Marlin S, McElreavey K, Sarkisian T, Grateau G, Alnemri ES, Amselem S. Mutations in NALP12 cause hereditary periodic fever syndromes. Proc Natl Acad Sci U S A. 2008;105:1614–1619. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

