The alpha2delta ligand gabapentin inhibits the Rab11-dependent recycling of the calcium channel subunit alpha2delta-2
- PMID: 20861389
- PMCID: PMC6633565
- DOI: 10.1523/JNEUROSCI.2700-10.2010
The alpha2delta ligand gabapentin inhibits the Rab11-dependent recycling of the calcium channel subunit alpha2delta-2
Abstract
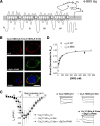
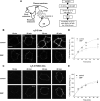
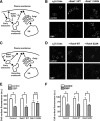
The α2δ subunits of voltage-gated calcium channels are important modulatory subunits that enhance calcium currents and may also have other roles in synaptogenesis. The antiepileptic and antiallodynic drug gabapentin (GBP) binds to the α2δ-1 and α2δ-2 isoforms of this protein, and its binding may disrupt the binding of an endogenous ligand, required for their correct function. We have shown previously that GBP produces a chronic inhibitory effect on calcium currents by causing a reduction in the total number of α2δ and α1 subunits at the cell surface. This action of GBP is likely to be attributable to a disruption of the trafficking of α2δ subunits, either to or from the plasma membrane. We studied the effect of GBP on the internalization of, and insertion into the plasma membrane of α2δ-2 using an α-bungarotoxin binding site-tagged α2δ-2 subunit, and a fluorescent derivative of α-bungarotoxin. We found that GBP specifically disrupts the insertion of α2δ-2 from post-Golgi compartments to the plasma membrane, and this effect was prevented by a mutation of α2δ-2 that abolishes its binding to GBP. The coexpression of the GDP-bound Rab11 S25N mutant prevented the GBP-induced decrease in α2δ-2 cell surface levels, both in cell lines and in primary neurons, and the GBP-induced reduction in calcium channel currents. In contrast, the internalization of α2δ-2 was unaffected by GBP. We conclude that GBP acts by preventing the recycling of α2δ-2 from Rab11-positive recycling endosomes to the plasma membrane.
Figures





Similar articles
-
Pharmacological disruption of calcium channel trafficking by the alpha2delta ligand gabapentin.Proc Natl Acad Sci U S A. 2008 Mar 4;105(9):3628-33. doi: 10.1073/pnas.0708930105. Epub 2008 Feb 25. Proc Natl Acad Sci U S A. 2008. PMID: 18299583 Free PMC article.
-
Gabapentinoid Insensitivity after Repeated Administration is Associated with Down-Regulation of the α(2)δ-1 Subunit in Rats with Central Post-Stroke Pain Hypersensitivity.Neurosci Bull. 2016 Feb;32(1):41-50. doi: 10.1007/s12264-015-0008-3. Epub 2016 Jan 19. Neurosci Bull. 2016. PMID: 26781878 Free PMC article.
-
Rab11-dependent recycling of calcium channels is mediated by auxiliary subunit α2δ-1 but not α2δ-3.Sci Rep. 2021 May 13;11(1):10256. doi: 10.1038/s41598-021-89820-1. Sci Rep. 2021. PMID: 33986433 Free PMC article.
-
Functional biology of the alpha(2)delta subunits of voltage-gated calcium channels.Trends Pharmacol Sci. 2007 May;28(5):220-8. doi: 10.1016/j.tips.2007.03.005. Epub 2007 Apr 2. Trends Pharmacol Sci. 2007. PMID: 17403543 Review.
-
Calcium channel alpha2delta subunits: differential expression, function, and drug binding.J Bioenerg Biomembr. 2003 Dec;35(6):639-47. doi: 10.1023/b:jobb.0000008028.41056.58. J Bioenerg Biomembr. 2003. PMID: 15000524 Review.
Cited by
-
Non-medical Use of Prescription Gabapentinoids (Gabapentin and Pregabalin) in Five European Countries.Front Psychiatry. 2021 Apr 28;12:676224. doi: 10.3389/fpsyt.2021.676224. eCollection 2021. Front Psychiatry. 2021. PMID: 33995154 Free PMC article.
-
Dynamic CRMP2 Regulation of CaV2.2 in the Prefrontal Cortex Contributes to the Reinstatement of Cocaine Seeking.Mol Neurobiol. 2020 Jan;57(1):346-357. doi: 10.1007/s12035-019-01711-9. Epub 2019 Jul 29. Mol Neurobiol. 2020. PMID: 31359322 Free PMC article.
-
Gabapentin and pregabalin in bipolar disorder, anxiety states, and insomnia: Systematic review, meta-analysis, and rationale.Mol Psychiatry. 2022 Mar;27(3):1339-1349. doi: 10.1038/s41380-021-01386-6. Epub 2021 Nov 24. Mol Psychiatry. 2022. PMID: 34819636 Free PMC article.
-
Six cases of (severe) hypoglycaemia associated with gabapentin use in both diabetic and non-diabetic patients.Br J Clin Pharmacol. 2015 May;79(5):870-1. doi: 10.1111/bcp.12548. Br J Clin Pharmacol. 2015. PMID: 25387462 Free PMC article. No abstract available.
-
Active Zone Trafficking of CaV2/UNC-2 Channels Is Independent of β/CCB-1 and α2δ/UNC-36 Subunits.J Neurosci. 2023 Jul 12;43(28):5142-5157. doi: 10.1523/JNEUROSCI.2264-22.2023. Epub 2023 May 9. J Neurosci. 2023. PMID: 37160370 Free PMC article.
References
-
- Alden KJ, García J. Differential effect of gabapentin on neuronal and muscle calcium currents. J Pharmacol Exp Ther. 2001;297:727–735. - PubMed
-
- Arikkath J, Campbell KP. Auxiliary subunits: essential components of the voltage-gated calcium channel complex. Curr Opin Neurobiol. 2003;13:298–307. - PubMed
-
- Barclay J, Balaguero N, Mione M, Ackerman SL, Letts VA, Brodbeck J, Canti C, Meir A, Page KM, Kusumi K, Perez-Reyes E, Lander ES, Frankel WN, Gardiner RM, Dolphin AC, Rees M. Ducky mouse phenotype of epilepsy and ataxia is associated with mutations in the Cacna2d2 gene and decreased calcium channel current in cerebellar Purkinje cells. J Neurosci. 2001;21:6095–6104. - PMC - PubMed
-
- Bauer CS, Nieto-Rostro M, Rahman W, Tran-Van-Minh A, Ferron L, Douglas L, Kadurin I, Sri Ranjan Y, Fernandez-Alacid L, Millar NS, Dickenson AH, Lujan R, Dolphin AC. The increased trafficking of the calcium channel α2δ-1 to presynaptic terminals in neuropathic pain is inhibited by the α2δ ligand pregabalin. J Neurosci. 2009;29:4076–4088. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
