Identification and functional characterization of malignant hyperthermia mutation T1354S in the outer pore of the Cavalpha1S-subunit
- PMID: 20861472
- PMCID: PMC3006335
- DOI: 10.1152/ajpcell.00008.2010
Identification and functional characterization of malignant hyperthermia mutation T1354S in the outer pore of the Cavalpha1S-subunit
Abstract
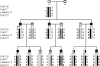
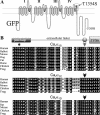
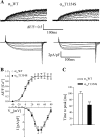
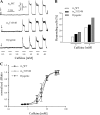
To identify the genetic locus responsible for malignant hyperthermia susceptibility (MHS) in an Italian family, we performed linkage analysis to recognized MHS loci. All MHS individuals showed cosegregation of informative markers close to the voltage-dependent Ca(2+) channel (Ca(V)) α(1S)-subunit gene (CACNA1S) with logarithm of odds (LOD)-score values that matched or approached the maximal possible value for this family. This is particularly interesting, because so far MHS was mapped to >178 different positions on the ryanodine receptor (RYR1) gene but only to two on CACNA1S. Sequence analysis of CACNA1S revealed a c.4060A>T transversion resulting in amino acid exchange T1354S in the IVS5-S6 extracellular pore-loop region of Ca(V)α(1S) in all MHS subjects of the family but not in 268 control subjects. To investigate the impact of mutation T1354S on the assembly and function of the excitation-contraction coupling apparatus, we expressed GFP-tagged α(1S)T1354S in dysgenic (α(1S)-null) myotubes. Whole cell patch-clamp analysis revealed that α(1S)T1354S produced significantly faster activation of L-type Ca(2+) currents upon 200-ms depolarizing test pulses compared with wild-type GFP-α(1S) (α(1S)WT). In addition, α(1S)T1354S-expressing myotubes showed a tendency to increased sensitivity for caffeine-induced Ca(2+) release and to larger action-potential-induced intracellular Ca(2+) transients under low (≤ 2 mM) caffeine concentrations compared with α(1S)WT. Thus our data suggest that an additional influx of Ca(2+) due to faster activation of the α(1S)T1354S L-type Ca(2+) current, in concert with higher caffeine sensitivity of Ca(2+) release, leads to elevated muscle contraction under pharmacological trigger, which might be sufficient to explain the MHS phenotype.
Figures

References
-
- Adams BA, Tanabe T, Mikami A, Numa S, Beam KG. Intramembrane charge movement restored in dysgenic skeletal muscle by injection of dihydropyridine receptor cDNAs. Nature 346: 569–572, 1990 - PubMed
-
- Bers DM. Cardiac excitation-contraction coupling. Nature 415: 198–205, 2002 - PubMed
-
- Caffrey JM. Kinetic properties of skeletal-muscle-like high-threshold calcium currents in a non-fusing muscle cell line. Pflügers Arch 427: 277–288, 1994 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

