An ADAMTSL2 founder mutation causes Musladin-Lueke Syndrome, a heritable disorder of beagle dogs, featuring stiff skin and joint contractures
- PMID: 20862248
- PMCID: PMC2941456
- DOI: 10.1371/journal.pone.0012817
An ADAMTSL2 founder mutation causes Musladin-Lueke Syndrome, a heritable disorder of beagle dogs, featuring stiff skin and joint contractures
Abstract
Background: Musladin-Lueke Syndrome (MLS) is a hereditary disorder affecting Beagle dogs that manifests with extensive fibrosis of the skin and joints. In this respect, it resembles human stiff skin syndrome and the Tight skin mouse, each of which is caused by gene defects affecting fibrillin-1, a major component of tissue microfibrils. The objective of this work was to determine the genetic basis of MLS and the molecular consequence of the identified mutation.
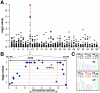
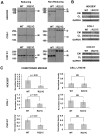
Methodology and principal findings: We mapped the locus for MLS by genome-wide association to a 3.05 Mb haplotype on canine chromosome 9 (CFA9 (50.11-54.26; p(raw) <10(-7))), which was homozygous and identical-by-descent among all affected dogs, consistent with recessive inheritance of a founder mutation. Sequence analysis of a candidate gene at this locus, ADAMTSL2, which is responsible for the human TGFβ dysregulation syndrome, Geleophysic Dysplasia (GD), uncovered a mutation in exon 7 (c.660C>T; p.R221C) perfectly associated with MLS (p-value=10(-12)). Murine ADAMTSL2 containing the p.R221C mutation formed anomalous disulfide-bonded dimers when transiently expressed in COS-1, HEK293F and CHO cells, and was present in the medium of these cells at lower levels than wild-type ADAMTSL2 expressed in parallel.
Conclusions/significance: The genetic basis of MLS is a founder mutation in ADAMTSL2, previously shown to interact with latent TGF-β binding protein, which binds fibrillin-1. The molecular effect of the founder mutation on ADAMTSL2 is formation of disulfide-bonded dimers. Although caused by a distinct mutation, and having a milder phenotype than human GD, MLS nevertheless offers a new animal model for study of GD, and for prospective insights on mechanisms and pathways of skin fibrosis and joint contractures.
Conflict of interest statement
Figures

References
-
- Annes JP, Munger JS, Rifkin DB. Making sense of latent TGFbeta activation. J Cell Sci. 2003;116:217–224. - PubMed
-
- Rifkin DB. Latent transforming growth factor-beta (TGF-beta) binding proteins: orchestrators of TGF-beta availability. J Biol Chem. 2005;280:7409–7412. - PubMed
-
- Isogai Z, Ono RN, Ushiro S, Keene DR, Chen Y, et al. Latent transforming growth factor beta-binding protein 1 interacts with fibrillin and is a microfibril-associated protein. J Biol Chem. 2003;278:2750–2757. - PubMed
-
- Dallas SL, Keene DR, Bruder SP, Saharinen J, Sakai LY, et al. Role of the latent transforming growth factor beta binding protein 1 in fibrillin-containing microfibrils in bone cells in vitro and in vivo. J Bone Miner Res. 2000;15:68–81. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

