Analysis of human and mouse reprogramming of somatic cells to induced pluripotent stem cells. What is in the plate?
- PMID: 20862250
- PMCID: PMC2941458
- DOI: 10.1371/journal.pone.0012664
Analysis of human and mouse reprogramming of somatic cells to induced pluripotent stem cells. What is in the plate?
Abstract
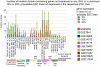
After the hope and controversy brought by embryonic stem cells two decades ago for regenerative medicine, a new turn has been taken in pluripotent cells research when, in 2006, Yamanaka's group reported the reprogramming of fibroblasts to pluripotent cells with the transfection of only four transcription factors. Since then many researchers have managed to reprogram somatic cells from diverse origins into pluripotent cells, though the cellular and genetic consequences of reprogramming remain largely unknown. Furthermore, it is still unclear whether induced pluripotent stem cells (iPSCs) are truly functionally equivalent to embryonic stem cells (ESCs) and if they demonstrate the same differentiation potential as ESCs. There are a large number of reprogramming experiments published so far encompassing genome-wide transcriptional profiling of the cells of origin, the iPSCs and ESCs, which are used as standards of pluripotent cells and allow us to provide here an in-depth analysis of transcriptional profiles of human and mouse cells before and after reprogramming. When compared to ESCs, iPSCs, as expected, share a common pluripotency/self-renewal network. Perhaps more importantly, they also show differences in the expression of some genes. We concentrated our efforts on the study of bivalent domain-containing genes (in ESCs) which are not expressed in ESCs, as they are supposedly important for differentiation and should possess a poised status in pluripotent cells, i.e. be ready to but not yet be expressed. We studied each iPSC line separately to estimate the quality of the reprogramming and saw a correlation of the lowest number of such genes expressed in each respective iPSC line with the stringency of the pluripotency test achieved by the line. We propose that the study of expression of bivalent domain-containing genes, which are normally silenced in ESCs, gives a valuable indication of the quality of the iPSC line, and could be used to select the best iPSC lines out of a large number of lines generated in each reprogramming experiment.
Conflict of interest statement
Figures



References
-
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. - PubMed
-
- Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–317. - PubMed
-
- Nakagawa M, Koyanagi M, Tanabe K, Takahashi K, Ichisaka T, et al. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol. 2008;26:101–106. - PubMed
-
- Wernig M, Meissner A, Cassady JP, Jaenisch R. c-Myc is dispensable for direct reprogramming of mouse fibroblasts. Cell Stem Cell. 2008;2:10–12. - PubMed
-
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

