Sub-nanoscale surface ruggedness provides a water-tight seal for exposed regions in soluble protein structure
- PMID: 20862253
- PMCID: PMC2941462
- DOI: 10.1371/journal.pone.0012844
Sub-nanoscale surface ruggedness provides a water-tight seal for exposed regions in soluble protein structure
Erratum in
- PLoS One. 2013;8(12). doi:10.1371/annotation/c17f2565-d129-4bb6-bb94-790ca1c6bb61
Abstract
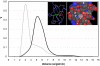
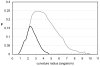
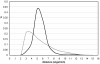
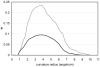
Soluble proteins must maintain backbone hydrogen bonds (BHBs) water-tight to ensure structural integrity. This protection is often achieved by burying the BHBs or wrapping them through intermolecular associations. On the other hand, water has low coordination resilience, with loss of hydrogen-bonding partnerships carrying significant thermodynamic cost. Thus, a core problem in structural biology is whether natural design actually exploits the water coordination stiffness to seal the backbone in regions that are exposed to the solvent. This work explores the molecular design features that make this type of seal operative, focusing on the side-chain arrangements that shield the protein backbone. We show that an efficient sealing is achieved by adapting the sub-nanoscale surface topography to the stringency of water coordination: an exposed BHB may be kept dry if the local concave curvature is small enough to impede formation of the coordination shell of a penetrating water molecule. Examination of an exhaustive database of uncomplexed proteins reveals that exposed BHBs invariably occur within such sub-nanoscale cavities in native folds, while this level of local ruggedness is absent in other regions. By contrast, BHB exposure in misfolded proteins occurs with larger local curvature promoting backbone hydration and consequently, structure disruption. These findings unravel physical constraints fitting a spatially dependent least-action for water coordination, introduce a molecular design concept, and herald the advent of water-tight peptide-based materials with sufficient backbone exposure to remain flexible.
Conflict of interest statement
Figures

References
-
- Fernández A. Transformative Concepts for Drug Design: Target Wrapping. Heidelberg: Springer; 2010.
-
- Baldwin RL. In search of the energetic role of peptide hydrogen bonds. J Biol Chem. 2003;278:17581–17588. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

