Bone is not essential for osteoclast activation
- PMID: 20862258
- PMCID: PMC2941467
- DOI: 10.1371/journal.pone.0012837
Bone is not essential for osteoclast activation
Abstract
Background: The mechanism whereby bone activates resorptive behavior in osteoclasts, the cells that resorb bone, is unknown. It is known that α(v)β(3) ligands are important, because blockade of α(v)β(3) receptor signaling inhibits bone resorption, but this might be through inhibition of adhesion or migration rather than resorption itself. Nor is it known whether α(v)β(3) ligands are sufficient for resorption the consensus is that bone mineral is essential for the recognition of bone as the substrate appropriate for resorption.
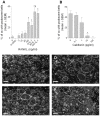
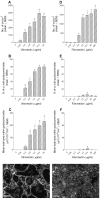
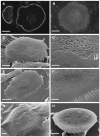
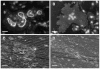
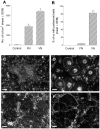
Methodology/principal findings: Vitronectin- but not fibronectin-coated coverslips induced murine osteoclasts to secrete tartrate-resistant acid phosphatase, as they do on bone. Osteoclasts incubated on vitronectin, unlike fibronectin, formed podosome belts on glass coverslips, and these were modulated by resorption-regulating cytokines. Podosome belts formed on vitronectin-coated surfaces whether the substrates were rough or smooth, rigid or flexible. We developed a novel approach whereby the substrate-apposed surface of cells can be visualized in the scanning electron microscope. With this approach, supported by transmission electron microscopy, we found that osteoclasts on vitronectin-coated surfaces show ruffled borders and clear zones characteristic of resorbing osteoclasts. Ruffles were obscured by a film if cells were incubated in the cathepsin inhibitor E64, suggesting that removal of the film represents substrate-degrading behavior. Analogously, osteoclasts formed resorption-like trails on vitronectin-coated substrates. Like bone resorption, these trails were dependent upon resorbogenic cytokines and were inhibited by E64. Bone mineral induced actin rings and surface excavation only if first coated with vitronectin. Fibronectin could not substitute in any of these activities, despite enabling adhesion and cell spreading.
Conclusions/significance: Our results show that ligands α(v)β(3) are not only necessary but sufficient for the induction of resorptive behavior in osteoclasts; and suggest that bone is recognized through its affinity for these ligands, rather than by its mechanical or topographical attributes, or through a putative 'mineral receptor'.
Conflict of interest statement
Figures




Similar articles
-
How are osteoclasts induced to resorb bone?Ann N Y Acad Sci. 2011 Dec;1240:1-6. doi: 10.1111/j.1749-6632.2011.06249.x. Ann N Y Acad Sci. 2011. PMID: 22172032 Review.
-
Secretion of tartrate-resistant acid phosphatase by osteoclasts correlates with resorptive behavior.J Cell Biochem. 2006 Aug 1;98(5):1085-94. doi: 10.1002/jcb.20835. J Cell Biochem. 2006. PMID: 16475168
-
Polarized osteoclasts put marks of tartrate-resistant acid phosphatase on dentin slices--a simple method for identifying polarized osteoclasts.Bone. 2011 Dec;49(6):1331-9. doi: 10.1016/j.bone.2011.09.045. Epub 2011 Sep 29. Bone. 2011. PMID: 21983021
-
The Foreign Body Giant Cell Cannot Resorb Bone, But Dissolves Hydroxyapatite Like Osteoclasts.PLoS One. 2015 Oct 1;10(10):e0139564. doi: 10.1371/journal.pone.0139564. eCollection 2015. PLoS One. 2015. PMID: 26426806 Free PMC article.
-
Integrin function in osteoclasts.J Endocrinol. 1997 Sep;154 Suppl:S47-56. J Endocrinol. 1997. PMID: 9379137 Review.
Cited by
-
The deep-sea natural products, biogenic polyphosphate (Bio-PolyP) and biogenic silica (Bio-Silica), as biomimetic scaffolds for bone tissue engineering: fabrication of a morphogenetically-active polymer.Mar Drugs. 2013 Mar 8;11(3):718-46. doi: 10.3390/md11030718. Mar Drugs. 2013. PMID: 23528950 Free PMC article. Review.
-
Multifunctional role of osteopontin in directing intrafibrillar mineralization of collagen and activation of osteoclasts.Acta Biomater. 2014 Jan;10(1):494-507. doi: 10.1016/j.actbio.2013.10.010. Epub 2013 Oct 17. Acta Biomater. 2014. PMID: 24140612 Free PMC article.
-
Podosome organization drives osteoclast-mediated bone resorption.Cell Adh Migr. 2014;8(3):191-204. doi: 10.4161/cam.27840. Cell Adh Migr. 2014. PMID: 24714644 Free PMC article. Review.
-
Vitamin D endocrine system and osteoclasts.Bonekey Rep. 2014 Feb 5;3:495. doi: 10.1038/bonekey.2013.229. eCollection 2014. Bonekey Rep. 2014. PMID: 24605212 Free PMC article.
-
Regulatory properties of vitronectin and its glycosylation in collagen fibril formation and collagen-degrading enzyme cathepsin K activity.Sci Rep. 2021 Jun 8;11(1):12023. doi: 10.1038/s41598-021-91353-6. Sci Rep. 2021. PMID: 34103584 Free PMC article.
References
-
- Chambers TJ. Regulation of the differentiation and function of osteoclasts. J Pathol. 2000;192:4–13. - PubMed
-
- Suda T, Takahashi N, Udagawa N, Jimi E, Gillespie MT, et al. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr Rev. 1999;20:345–357. - PubMed
-
- Vaananen HK, Zhao H, Mulari M, Halleen JM. The cell biology of osteoclast function. J Cell Sci. 2000;113(Pt 3):377–381. - PubMed
-
- Stenbeck G. Formation and function of the ruffled border in osteoclasts. Semin Cell Dev Biol. 2002;13:285–292. - PubMed
-
- Fisher JE, Caulfield MP, Sato M, Quartuccio HA, Gould RJ, et al. Inhibition of Osteoclastic Bone-Resorption Invivo by Echistatin, an Arginyl-Glycyl-Aspartyl (Rgd)-Containing Protein. Endocrinology. 1993;132:1411–1413. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

