Targeted p120-catenin ablation disrupts dental enamel development
- PMID: 20862276
- PMCID: PMC2940824
- DOI: 10.1371/journal.pone.0012703
Targeted p120-catenin ablation disrupts dental enamel development
Abstract
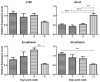
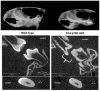
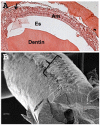
Dental enamel development occurs in stages. The ameloblast cell layer is adjacent to, and is responsible for, enamel formation. When rodent pre-ameloblasts become tall columnar secretory-stage ameloblasts, they secrete enamel matrix proteins, and the ameloblasts start moving in rows that slide by one another. This movement is necessary to form the characteristic decussating enamel prism pattern. Thus, a dynamic system of intercellular interactions is required for proper enamel development. Cadherins are components of the adherens junction (AJ), and they span the cell membrane to mediate attachment to adjacent cells. p120 stabilizes cadherins by preventing their internalization and degradation. So, we asked if p120-mediated cadherin stability is important for dental enamel formation. Targeted p120 ablation in the mouse enamel organ had a striking effect. Secretory stage ameloblasts detached from surrounding tissues, lost polarity, flattened, and ameloblast E- and N-cadherin expression became undetectable by immunostaining. The enamel itself was poorly mineralized and appeared to be composed of a thin layer of merged spheres that abraded from the tooth. Significantly, p120 mosaic mouse teeth were capable of forming normal enamel demonstrating that the enamel defects were not a secondary effect of p120 ablation. Surprisingly, blood-filled sinusoids developed in random locations around the developing teeth. This has not been observed in other p120-ablated tissues and may be due to altered p120-mediated cell signaling. These data reveal a critical role for p120 in tooth and dental enamel development and are consistent with p120 directing the attachment and detachment of the secretory stage ameloblasts as they move in rows.
Conflict of interest statement
Figures






References
-
- Leblond CP, Warshawsky H. Dynamics of enamel formation in the rat incisor tooth. J Dent Res. 1979;58:950–975. - PubMed
-
- Warshawsky H, Smith CE. A three-dimensional reconstruction of the rods in rat maxillary incisor enamel. Anat Rec. 1971;169:585–591. - PubMed
-
- Nishikawa S, Fujiwara K, Kitamura H. Formation of the tooth enamel rod pattern and the cytoskeletal organization in secretory ameloblasts of the rat incisor. Eur J Cell Biol. 1988;47:222–232. - PubMed
-
- Reith EJ, Ross MH. Morphological evidence for the presence of contractile elements in secretory ameloblasts of the rat. Arch Oral Biol. 1973;18:445–448. - PubMed
-
- Risnes S. A method of calculating the speed of movement of ameloblasts during rat incisor amelogenesis. Arch Oral Biol. 1979;24:299–306. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

