Generation of genic diversity among Streptococcus pneumoniae strains via horizontal gene transfer during a chronic polyclonal pediatric infection
- PMID: 20862314
- PMCID: PMC2940740
- DOI: 10.1371/journal.ppat.1001108
Generation of genic diversity among Streptococcus pneumoniae strains via horizontal gene transfer during a chronic polyclonal pediatric infection
Abstract
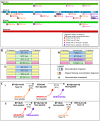
Although there is tremendous interest in understanding the evolutionary roles of horizontal gene transfer (HGT) processes that occur during chronic polyclonal infections, to date there have been few studies that directly address this topic. We have characterized multiple HGT events that most likely occurred during polyclonal infection among nasopharyngeal strains of Streptococcus pneumoniae recovered from a child suffering from chronic upper respiratory and middle-ear infections. Whole genome sequencing and comparative genomics were performed on six isolates collected during symptomatic episodes over a period of seven months. From these comparisons we determined that five of the isolates were genetically highly similar and likely represented a dominant lineage. We analyzed all genic and allelic differences among all six isolates and found that all differences tended to occur within contiguous genomic blocks, suggestive of strain evolution by homologous recombination. From these analyses we identified three strains (two of which were recovered on two different occasions) that appear to have been derived sequentially, one from the next, each by multiple recombination events. We also identified a fourth strain that contains many of the genomic segments that differentiate the three highly related strains from one another, and have hypothesized that this fourth strain may have served as a donor multiple times in the evolution of the dominant strain line. The variations among the parent, daughter, and grand-daughter recombinant strains collectively cover greater than seven percent of the genome and are grouped into 23 chromosomal clusters. While capturing in vivo HGT, these data support the distributed genome hypothesis and suggest that a single competence event in pneumococci can result in the replacement of DNA at multiple non-adjacent loci.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

