Polymorphisms in Anopheles gambiae immune genes associated with natural resistance to Plasmodium falciparum
- PMID: 20862317
- PMCID: PMC2940751
- DOI: 10.1371/journal.ppat.1001112
Polymorphisms in Anopheles gambiae immune genes associated with natural resistance to Plasmodium falciparum
Abstract
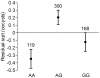
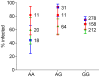
Many genes involved in the immune response of Anopheles gambiae, the main malaria vector in Africa, have been identified, but whether naturally occurring polymorphisms in these genes underlie variation in resistance to the human malaria parasite, Plasmodium falciparum, is currently unknown. Here we carried out a candidate gene association study to identify single nucleotide polymorphisms (SNPs) associated with natural resistance to P. falciparum. A. gambiae M form mosquitoes from Cameroon were experimentally challenged with three local wild P. falciparum isolates. Statistical associations were assessed between 157 SNPs selected from a set of 67 A. gambiae immune-related genes and the level of infection. Isolate-specific associations were accounted for by including the effect of the isolate in the analysis. Five SNPs were significantly associated to the infection phenotype, located within or upstream of AgMDL1, CEC1, Sp PPO activate, Sp SNAKElike, and TOLL6. Low overall and local linkage disequilibrium indicated high specificity in the loci found. Association between infection phenotype and two SNPs was isolate-specific, providing the first evidence of vector genotype by parasite isolate interactions at the molecular level. Four SNPs were associated to either oocyst presence or load, indicating that the genetic basis of infection prevalence and intensity may differ. The validity of the approach was verified by confirming the functional role of Sp SNAKElike in gene silencing assays. These results strongly support the role of genetic variation within or near these five A. gambiae immune genes, in concert with other genes, in natural resistance to P. falciparum. They emphasize the need to distinguish between infection prevalence and intensity and to account for the genetic specificity of vector-parasite interactions in dissecting the genetic basis of Anopheles resistance to human malaria.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Identification of three single nucleotide polymorphisms in Anopheles gambiae immune signaling genes that are associated with natural Plasmodium falciparum infection.Malar J. 2010 Jun 11;9:160. doi: 10.1186/1475-2875-9-160. Malar J. 2010. PMID: 20540770 Free PMC article.
-
Association mapping by pooled sequencing identifies TOLL 11 as a protective factor against Plasmodium falciparum in Anopheles gambiae.BMC Genomics. 2015 Oct 13;16:779. doi: 10.1186/s12864-015-2009-z. BMC Genomics. 2015. PMID: 26462916 Free PMC article.
-
Host genotype by parasite genotype interactions underlying the resistance of anopheline mosquitoes to Plasmodium falciparum.Malar J. 2005 Jan 11;4:3. doi: 10.1186/1475-2875-4-3. Malar J. 2005. PMID: 15644136 Free PMC article.
-
Innate immunity in the malaria vector Anopheles gambiae: comparative and functional genomics.J Exp Biol. 2004 Jul;207(Pt 15):2551-63. doi: 10.1242/jeb.01066. J Exp Biol. 2004. PMID: 15201288 Review.
-
Genetic basis of encapsulation response in Anopheles gambiae.Parassitologia. 1999 Sep;41(1-3):181-4. Parassitologia. 1999. PMID: 10697853 Review.
Cited by
-
Diverged alleles of the Anopheles gambiae leucine-rich repeat gene APL1A display distinct protective profiles against Plasmodium falciparum.PLoS One. 2012;7(12):e52684. doi: 10.1371/journal.pone.0052684. Epub 2012 Dec 28. PLoS One. 2012. PMID: 23285147 Free PMC article.
-
Impact of field-realistic doses of glyphosate and nutritional stress on mosquito life history traits and susceptibility to malaria parasite infection.Ecol Evol. 2020 Apr 27;10(11):5079-5088. doi: 10.1002/ece3.6261. eCollection 2020 Jun. Ecol Evol. 2020. PMID: 32551083 Free PMC article.
-
Susceptibility of Field-Collected Nyssorhynchus darlingi to Plasmodium spp. in Western Amazonian Brazil.Genes (Basel). 2021 Oct 25;12(11):1693. doi: 10.3390/genes12111693. Genes (Basel). 2021. PMID: 34828299 Free PMC article.
-
Asaia accelerates larval development of Anopheles gambiae.Pathog Glob Health. 2013 Sep;107(6):305-11. doi: 10.1179/2047773213Y.0000000106. Epub 2013 Jul 26. Pathog Glob Health. 2013. PMID: 24091152 Free PMC article.
-
Intervention reducing malaria parasite load in vector mosquitoes: No impact on Plasmodium falciparum extrinsic incubation period and the survival of Anopheles gambiae.PLoS Pathog. 2023 May 17;19(5):e1011084. doi: 10.1371/journal.ppat.1011084. eCollection 2023 May. PLoS Pathog. 2023. PMID: 37195964 Free PMC article.
References
-
- Sinden RE, Alavi Y, Raine JD. Mosquito—malaria interactions: a reappraisal of the concepts of susceptibility and refractoriness. Insect Biochem Mol Biol. 2004;34:625–629. - PubMed
-
- Collins FH, Sakai RK, Vernick KD, Paskewitz S, Seeley DC, et al. Genetic selection of a Plasmodium-refractory strain of the malaria vector Anopheles gambiae. Science. 1986;234:607–610. - PubMed
-
- Vernick KD, Fujioka H, Seeley DC, Tandler B, Aikawa M, et al. Plasmodium gallinaceum: a refractory mechanism of ookinete killing in the mosquito, Anopheles gambiae. Exp Parasitol. 1995;80:583–595. - PubMed
-
- Christophides GK. Transgenic mosquitoes and malaria transmission. Cell Microbiol. 2005;7:325–333. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

