Assembly of filopodia by the formin FRL2 (FMNL3)
- PMID: 20862687
- PMCID: PMC2991502
- DOI: 10.1002/cm.20485
Assembly of filopodia by the formin FRL2 (FMNL3)
Abstract
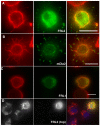
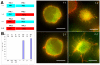
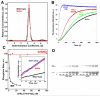
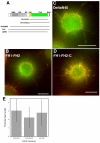
Actin-dependent finger-like protrusions such as filopodia and microvilli are widespread in eukaryotes, but their assembly mechanisms are poorly understood. Filopodia assembly requires at least three biochemical activities on actin: actin filament nucleation, prolonged actin filament elongation, and actin filament bundling. These activities are shared by several mammalian formin proteins, including mDia2, FRL1 (also called FMNL1), and FRL2 (FMNL3). In this paper, we compare the abilities of constructs from these three formins to induce filopodia. FH1-FH2 constructs of both FRL2 and mDia2 stimulate potent filopodia assembly in multiple cell types, and enrich strongly at filopodia tips. In contrast, FRL1 FH1-FH2 lacks this activity, despite possessing similar biochemical activities and being highly homologous to FRL2. Chimeric FH1-FH2 experiments between FRL1 and FRL2 show that, while both an FH1 and an FH2 are needed, either FH1 domain supports filopodia assembly but only FRL2's FH2 domain allows this activity. A mutation that compromises FRL2's barbed end binding ability abolishes filopodia assembly. FRL2's ability to stimulate filopodia assembly is not altered by additional domains (GBD, DID, DAD), but is significantly reduced in the full-length construct, suggesting that FRL2 is subject to inhibitory regulation. The data suggest that the FH2 domain of FRL2 possesses properties not shared by FRL1 that allow it to generate filopodia.
Copyright © 2010 Wiley-Liss, Inc.
Figures





References
-
- Berg JS, Derfler BH, Pennisi CM, Corey DP, Cheney RE. Myosin-X, a novel myosin with pleckstrin homology domains, associates with regions of dynamic actin. J Cell Sci. 2000;113(Pt 19):3439–3451. - PubMed
-
- Block J, Stradal TE, Hanisch J, Geffers R, Kostler SA, Urban E, Small JV, Rottner K, Faix J. Filopodia formation induced by active mDia2/Drf3. J Microsc. 2008;231(3):506–17. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

