A reexamination of active and passive tumor targeting by using rod-shaped gold nanocrystals and covalently conjugated peptide ligands
- PMID: 20863096
- PMCID: PMC2964428
- DOI: 10.1021/nn102055s
A reexamination of active and passive tumor targeting by using rod-shaped gold nanocrystals and covalently conjugated peptide ligands
Erratum in
- ACS Nano. 2011 Aug 23;5(8):6765
Abstract
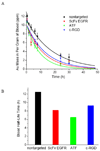
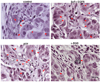
The targeted delivery of nanoparticles to solid tumors is one of the most important and challenging problems in cancer nanomedicine, but the detailed delivery mechanisms and design principles are still not well understood. Here we report quantitative tumor uptake studies for a class of elongated gold nanocrystals (called nanorods) that are covalently conjugated to tumor-targeting peptides. A major advantage in using gold as a "tracer" is that the accumulated gold in tumors and other organs can be quantitatively determined by elemental mass spectrometry (gold is not a natural element found in animals). Thus, colloidal gold nanorods are stabilized with a layer of polyethylene glycols (PEGs) and are conjugated to three different ligands: (i) a single-chain variable fragment (ScFv) peptide that recognizes the epidermal growth factor receptor (EGFR); (ii) an amino terminal fragment (ATF) peptide that recognizes the urokinase plasminogen activator receptor (uPAR); and (iii) a cyclic RGD peptide that recognizes the a(v)β(3) integrin receptor. Quantitative pharmacokinetic and biodistribution data show that these targeting ligands only marginally improve the total gold accumulation in xenograft tumor models in comparison with nontargeted controls, but their use could greatly alter the intracellular and extracellular nanoparticle distributions. When the gold nanorods are administered via intravenous injection, we also find that active molecular targeting of the tumor microenvironments (e.g., fibroblasts, macrophages, and vasculatures) does not significantly influence the tumor nanoparticle uptake. These results suggest that for photothermal cancer therapy, the preferred route of gold nanorod administration is intratumoral injection instead of intravenous injection.
Figures



References
-
- Ferrari M. Cancer Nanotechnology: Opportunities and Challenges. Nat. Rev. Cancer. 2005;5:161–171. - PubMed
-
- Farokhzad OC, Langer R. Nanomedicine: Developing Smarter Therapeutic and Diagnostic Modalities. Adv. Drug Del. Rev. 2006;58:1456–1459. - PubMed
-
- Nie S, Xing Y, Kim GJ, Simons JM. Nanotechnology Applications in Cancer. Annu. Rev. Biomed. Eng. 2007;9:257–288. - PubMed
-
- Sanhai WR, Sakamoto JH, Canady R, Ferrari M. Seven Challenges for Nanomedicine. Nat. Nanotechol. 2008;3:242–244. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

