Rapamycin inhibits cell proliferation in type I and type II endometrial carcinomas: a search for biomarkers of sensitivity to treatment
- PMID: 20863555
- PMCID: PMC4098865
- DOI: 10.1016/j.ygyno.2010.08.025
Rapamycin inhibits cell proliferation in type I and type II endometrial carcinomas: a search for biomarkers of sensitivity to treatment
Abstract
Objectives: Our goal was to evaluate the effect of rapamycin, an mTOR inhibitor, in type I and II human endometrial cancer tumor explants.
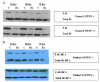
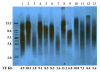
Methods: Short-term tissue culture with fresh endometrial cancer tumor explants was performed. Cell proliferation was assessed by MTS assay after treatment with rapamycin. Akt and PTEN status were documented by Western blotting. The effect of rapamycin on phosphorylated-S6 and 4E-BP-1 was also assessed by Western blotting. Real-time RT-PCR was used to quantify hTERT mRNA expression. Telomere length was determined by terminal restriction fragment Southern blotting.
Results: Thirteen fresh endometrial cancer tumor explants (nine Type I, four Type II) were placed in short-term culture and treated with rapamycin. Nine of the endometrial cancer tumors responded to rapamycin, with a median IC₅₀ of 11.4 nM. Sensitivity to rapamycin was independent of PTEN and Akt status. Tumors (13/13) had a reduction in phosphorylated-S6 and 10/13 had a reduction in phosphorylated 4E-BP-1. Rapamycin decreased hTERT mRNA expression in all of the endometrial cancer tumors. Telomere length did not correspond with responsiveness to this drug.
Conclusions: Rapamycin demonstrated activity in fresh endometrial tumor explants independent of PTEN and Akt status. Some tumors demonstrated a reduction in phosphorylated-S6 and 4E-BP-1 without a significant change in cellular proliferation, suggesting that additional pathways may modulate cellular proliferation. Thus, mTOR inhibitors may be a useful targeted therapy for both type I and type II endometrial cancers, but the search remains for a predictive biomarker of sensitivity to this therapy.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Rapamycin inhibits telomerase activity by decreasing the hTERT mRNA level in endometrial cancer cells.Mol Cancer Ther. 2003 Aug;2(8):789-95. Mol Cancer Ther. 2003. PMID: 12939469
-
Rapamycin inhibits hTERT telomerase mRNA expression, independent of cell cycle arrest.Gynecol Oncol. 2006 Mar;100(3):487-94. doi: 10.1016/j.ygyno.2005.08.053. Epub 2005 Oct 24. Gynecol Oncol. 2006. PMID: 16249016
-
Dual mTORC1/2 inhibition in a preclinical xenograft tumor model of endometrial cancer.Gynecol Oncol. 2014 Feb;132(2):468-73. doi: 10.1016/j.ygyno.2013.11.027. Epub 2013 Dec 4. Gynecol Oncol. 2014. PMID: 24316308 Free PMC article.
-
Molecular target therapies in endometrial cancer: from the basic research to the clinic.Gynecol Endocrinol. 2008 May;24(5):239-49. doi: 10.1080/09513590801953556. Gynecol Endocrinol. 2008. PMID: 18569027 Review.
-
Targeting the mTOR/4E-BP pathway in endometrial cancer.Clin Cancer Res. 2011 Dec 15;17(24):7518-28. doi: 10.1158/1078-0432.CCR-11-1664. Epub 2011 Dec 5. Clin Cancer Res. 2011. PMID: 22142830 Review.
Cited by
-
Progestin Resistance and Corresponding Management of Abnormal Endometrial Hyperplasia and Endometrial Carcinoma.Cancers (Basel). 2022 Dec 15;14(24):6210. doi: 10.3390/cancers14246210. Cancers (Basel). 2022. PMID: 36551694 Free PMC article. Review.
-
The two faces of miR-29.J Cardiovasc Med (Hagerstown). 2015 Jul;16(7):480-90. doi: 10.2459/JCM.0000000000000246. J Cardiovasc Med (Hagerstown). 2015. PMID: 25689084 Free PMC article. Review.
-
Predictive biomarkers for the activity of mammalian target of rapamycin (mTOR) inhibitors.Target Oncol. 2011 Jun;6(2):119-24. doi: 10.1007/s11523-011-0177-6. Epub 2011 Apr 28. Target Oncol. 2011. PMID: 21533544 Review.
-
Effectiveness of inhibitor rapamycin, saracatinib, linsitinib and JNJ-38877605 against human prostate cancer cells.Int J Clin Exp Med. 2015 Apr 15;8(4):6563-7. eCollection 2015. Int J Clin Exp Med. 2015. PMID: 26131286 Free PMC article.
-
Different toxic effects of YTX in tumor K-562 and lymphoblastoid cell lines.Front Pharmacol. 2015 Jun 17;6:124. doi: 10.3389/fphar.2015.00124. eCollection 2015. Front Pharmacol. 2015. PMID: 26136685 Free PMC article.
References
-
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59(4):225–49. - PubMed
-
- Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol. 1983;15(1):10–7. - PubMed
-
- Abal M, Planaguma J, Gil-Moreno A, et al. Molecular pathology of endometrial carcinoma: transcriptional signature in endometrioid tumors. Histol Histopathol. 2006;21(2):197–204. - PubMed
-
- Hecht JL, Mutter GL. Molecular and pathologic aspects of endometrial carcinogenesis. J Clin Oncol. 2006;24(29):4783–91. - PubMed
-
- Lax SF. Molecular genetic pathways in various types of endometrial carcinoma: from a phenotypical to a molecular-based classification. Virchows Arch. 2004;444(3):213–23. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

