Sirtuin regulation of mitochondria: energy production, apoptosis, and signaling
- PMID: 20863707
- PMCID: PMC2992946
- DOI: 10.1016/j.tibs.2010.07.003
Sirtuin regulation of mitochondria: energy production, apoptosis, and signaling
Abstract
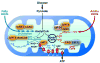
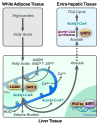
Sirtuins are a highly conserved family of proteins whose activity can prolong the lifespan of model organisms such as yeast, worms and flies. Mammals contain seven sirtuins (SIRT1-7) that modulate distinct metabolic and stress response pathways. Three sirtuins, SIRT3, SIRT4 and SIRT5, are located in the mitochondria, dynamic organelles that function as the primary site of oxidative metabolism and play crucial roles in apoptosis and intracellular signaling. Recent findings have shed light on how the mitochondrial sirtuins function in the control of basic mitochondrial biology, including energy production, metabolism, apoptosis and intracellular signaling.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures
References
-
- Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. - PubMed
-
- Ryan MT, Hoogenraad NJ. Mitochondrial-nuclear communications. Annu Rev Biochem. 2007;76:701–722. - PubMed
-
- Scarpulla RC. Nuclear activators and coactivators in mammalian mitochondrial biogenesis. Biochim Biophys Acta. 2002;1576:1–14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

