Characterization of Durham virus, a novel rhabdovirus that encodes both a C and SH protein
- PMID: 20863863
- PMCID: PMC3010432
- DOI: 10.1016/j.virusres.2010.09.007
Characterization of Durham virus, a novel rhabdovirus that encodes both a C and SH protein
Abstract
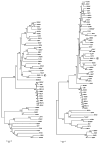
The family Rhabdoviridae is a diverse group of non-segmented, negative-sense RNA viruses that are distributed worldwide and infect a wide range of hosts including vertebrates, invertebrates, and plants. Of the 114 currently recognized vertebrate rhabdoviruses, relatively few have been well characterized at both the antigenic and genetic level; hence, the phylogenetic relationships between many of the vertebrate rhabdoviruses remain unknown. The present report describes a novel rhabdovirus isolated from the brain of a moribund American coot (Fulica americana) that exhibited neurological signs when found in Durham County, North Carolina, in 2005. Antigenic characterization of the virus revealed that it was serologically unrelated to 68 other known vertebrate rhabdoviruses. Genomic sequencing of the virus indicated that it shared the highest identity to Tupaia rhabdovirus (TUPV), and as only previously observed in TUPV, the genome encoded a putative C protein in an overlapping open reading frame (ORF) of the phosphoprotein gene and a small hydrophobic (SH) protein located in a novel ORF between the matrix and glycoprotein genes. Phylogenetic analysis of partial amino acid sequences of the nucleoprotein and polymerase protein indicated that, in addition to TUPV, the virus was most closely related to avian and small mammal rhabdoviruses from Africa and North America. In this report, we present the morphological, pathological, antigenic, and genetic characterization of the new virus, tentatively named Durham virus (DURV), and discuss its potential evolutionary relationship to other vertebrate rhabdoviruses.
Copyright © 2010 Elsevier B.V. All rights reserved.
Figures




References
-
- Blom N, Gammeltoft S, Brunak S. Sequence- and structure-based prediction of eukaryotic protein phosphorylation sites. J Mol Biol. 1999;294:1351–1362. - PubMed
-
- Bourhy H, Cowley JA, Larrous F, Holmes EC, Walker PG. Phylogenetic relationships among rhabdoviruses inferred using the L polymerase gene. J Gen Virol. 2005;86:2849–2858. - PubMed
-
- Calisher CH, Karabatsos N, Zeller H, Digoutte JP, Tesh RB, Shope RE, Travassos da Rosa AP, St George TD. Antigenic relationships among rhabdoviruses from vertebrates and hematophagous arthropods. Intervirology. 1989;30:241–257. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

