The dopamine D1-D2 receptor heteromer localizes in dynorphin/enkephalin neurons: increased high affinity state following amphetamine and in schizophrenia
- PMID: 20864528
- PMCID: PMC2978591
- DOI: 10.1074/jbc.M110.159954
The dopamine D1-D2 receptor heteromer localizes in dynorphin/enkephalin neurons: increased high affinity state following amphetamine and in schizophrenia
Abstract
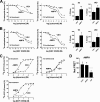
The distribution and function of neurons coexpressing the dopamine D1 and D2 receptors in the basal ganglia and mesolimbic system are unknown. We found a subset of medium spiny neurons coexpressing D1 and D2 receptors in varying densities throughout the basal ganglia, with the highest incidence in nucleus accumbens and globus pallidus and the lowest incidence in caudate putamen. These receptors formed D1-D2 receptor heteromers that were localized to cell bodies and presynaptic terminals. In rats, selective activation of D1-D2 heteromers increased grooming behavior and attenuated AMPA receptor GluR1 phosphorylation by calcium/calmodulin kinase IIα in nucleus accumbens, implying a role in reward pathways. D1-D2 heteromer sensitivity and functional activity was up-regulated in rat striatum by chronic amphetamine treatment and in globus pallidus from schizophrenia patients, indicating that the dopamine D1-D2 heteromer may contribute to psychopathologies of drug abuse, schizophrenia, or other disorders involving elevated dopamine transmission.
Figures






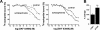
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

