A randomized trial of parental behavioral counseling and cotinine feedback for lowering environmental tobacco smoke exposure in children with asthma: results of the LET'S Manage Asthma trial
- PMID: 20864611
- PMCID: PMC3047287
- DOI: 10.1378/chest.10-0772
A randomized trial of parental behavioral counseling and cotinine feedback for lowering environmental tobacco smoke exposure in children with asthma: results of the LET'S Manage Asthma trial
Abstract
Background: Secondhand tobacco smoke exposure impairs the control of pediatric asthma. Evidence of the efficacy of interventions to reduce children's exposure and improve disease outcomes has been inconclusive.
Methods: Caregivers of 519 children aged 3 to 12 years with asthma and reported smoke exposure attended two baseline assessment visits, which involved a parent interview, sampling of the children's urine (for cotinine assay), and spirometry (children≥5 years). The caregivers and children (n=352) with significant documented exposure (cotinine≥10 ng/mL) attended a basic asthma education session, provided a third urine sample, and were randomized to the Lowering Environmental Tobacco Smoke: LET'S Manage Asthma (LET'S) intervention (n=178) or usual care (n=174). LET'S included three in-person, stage-of-change-based counseling sessions plus three follow-up phone calls. Cotinine feedback was given at each in-person session. Follow-up visits at 6 and 12 months postrandomization repeated the baseline data collection. Multivariate regression analyses estimated the intervention effect on the natural logarithm of the cotinine to creatinine ratio (lnCCR), use of health-care services, and other outcomes.
Results: In the sample overall, the children in the LET'S intervention had lower follow-up lnCCR values compared with the children in usual care, but the group difference was not significant (β coefficient=-0.307, P=.064), and there was no group difference in the odds of having>one asthma-related medical visit (β coefficient=0.035, P=.78). However, children with high-risk asthma had statistically lower follow-up lnCCR values compared with children in usual care (β coefficient=-1.068, P=.006).
Conclusions: The LET'S intervention was not associated with a statistically significant reduction in tobacco smoke exposure or use of health-care services in the sample as a whole. However, it appeared effective in reducing exposure in children at high risk for subsequent exacerbations.
Trial registry: ClinicialTrials.gov; No.: NCT00217958; URL: clinicaltrials.gov.
Figures
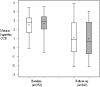
 = LET’S intervention group. CCR = cotinine to creatinine ratio; lnCCR = natural logarithm of the cotinine to creatinine ratio. See Figure 1 legend for expansion of the other abbreviation.
= LET’S intervention group. CCR = cotinine to creatinine ratio; lnCCR = natural logarithm of the cotinine to creatinine ratio. See Figure 1 legend for expansion of the other abbreviation.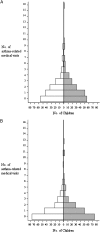
 = LET’S intervention group. See Figure 1 and 2 legends for expansion of the abbreviations.
= LET’S intervention group. See Figure 1 and 2 legends for expansion of the abbreviations.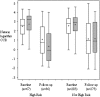
 = LET’S intervention group. See Figures 1 and 2 legends for expansion of the abbreviations.
= LET’S intervention group. See Figures 1 and 2 legends for expansion of the abbreviations.Similar articles
-
Secondhand tobacco smoke in children with asthma: sources of and parental perceptions about exposure in children and parental readiness to change.Chest. 2008 Jun;133(6):1367-1374. doi: 10.1378/chest.07-2369. Epub 2008 Mar 13. Chest. 2008. PMID: 18339788 Free PMC article. Clinical Trial.
-
Counseling to reduce children's secondhand smoke exposure and help parents quit smoking: a controlled trial.Nicotine Tob Res. 2009 Dec;11(12):1383-94. doi: 10.1093/ntr/ntp148. Epub 2009 Oct 29. Nicotine Tob Res. 2009. PMID: 19875762 Free PMC article. Clinical Trial.
-
A controlled trial of an environmental tobacco smoke reduction intervention in low-income children with asthma.Chest. 2001 Nov;120(5):1709-22. doi: 10.1378/chest.120.5.1709. Chest. 2001. PMID: 11713157 Clinical Trial.
-
Effectiveness of a School-Based 'Tobacco Free' Intervention on Adolescents' Knowledge and Exposure to Second Hand Tobacco Smoke - A Multiphase Study.Asian Pac J Cancer Prev. 2019 Dec 1;20(12):3533-3537. doi: 10.31557/APJCP.2019.20.12.3533. Asian Pac J Cancer Prev. 2019. PMID: 31870091 Free PMC article. Review.
-
Interventions by Health Care Professionals Who Provide Routine Child Health Care to Reduce Tobacco Smoke Exposure in Children: A Review and Meta-analysis.JAMA Pediatr. 2016 Feb;170(2):138-47. doi: 10.1001/jamapediatrics.2015.3342. JAMA Pediatr. 2016. PMID: 26719991 Review.
Cited by
-
Integrating asthma education and smoking cessation for parents: financial return on investment.Pediatr Pulmonol. 2012 Oct;47(10):950-5. doi: 10.1002/ppul.22559. Epub 2012 Mar 29. Pediatr Pulmonol. 2012. PMID: 22467563 Free PMC article.
-
Psychosocial factors and behavioral medicine interventions in asthma.J Consult Clin Psychol. 2013 Apr;81(2):231-50. doi: 10.1037/a0030187. Epub 2012 Oct 1. J Consult Clin Psychol. 2013. PMID: 23025250 Free PMC article.
-
A motivational interviewing intervention to PREvent PAssive Smoke Exposure (PREPASE) in children with a high risk of asthma: design of a randomised controlled trial.BMC Public Health. 2013 Feb 27;13:177. doi: 10.1186/1471-2458-13-177. BMC Public Health. 2013. PMID: 23442389 Free PMC article. Clinical Trial.
-
Motivational interviewing and urine cotinine feedback to stop passive smoke exposure in children predisposed to asthma: a randomised controlled trial.Sci Rep. 2017 Nov 13;7(1):15473. doi: 10.1038/s41598-017-15158-2. Sci Rep. 2017. PMID: 29133798 Free PMC article. Clinical Trial.
-
Does raising awareness in families reduce environmental tobacco smoke exposure in wheezy children?Postepy Dermatol Alergol. 2017 Aug;34(4):350-356. doi: 10.5114/ada.2017.69316. Epub 2017 Aug 2. Postepy Dermatol Alergol. 2017. PMID: 28951711 Free PMC article.
References
-
- Office of the Surgeon General . The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. Rockville, MD: US Dept of Health and Human Services, Public Health Service, Office of the Surgeon General; 2006.
-
- National Asthma Education and Prevention Program . Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. Bethesda, MD: National Institutes of Health; 2007. Publication No. 09-4051.
-
- Leong JW, Dore ND, Shelley K, et al. The elimination half-life of urinary cotinine in children of tobacco-smoking mothers. Pulm Pharmacol Ther. 1998;11(4):287–290. - PubMed

