Kaposi's sarcoma and its associated herpesvirus
- PMID: 20865011
- PMCID: PMC4721662
- DOI: 10.1038/nrc2888
Kaposi's sarcoma and its associated herpesvirus
Abstract
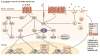
Kaposi's sarcoma (KS) is the most common cancer in HIV-infected untreated individuals. Kaposi's sarcoma-associated herpesvirus (KSHV; also known as human herpesvirus 8 (HHV8)) is the infectious cause of this neoplasm. In this Review we describe the epidemiology of KS and KSHV, and the insights into the remarkable mechanisms through which KSHV can induce KS that have been gained in the past 16 years. KSHV latent transcripts, such as latency-associated nuclear antigen (LANA), viral cyclin, viral FLIP and viral-encoded microRNAs, drive cell proliferation and prevent apoptosis, whereas KSHV lytic proteins, such as viral G protein-coupled receptor, K1 and virally encoded cytokines (viral interleukin-6 and viral chemokines) further contribute to the unique angioproliferative and inflammatory KS lesions through a mechanism called paracrine neoplasia.
Figures






References
-
- Kaposi M. Idiopathisches multiples Pigmentsarkom der Haut. Arch Dermatol Syph. 1872;4 Original description of KS.
-
- Centers for Disease Control. Kaposi’s sarcoma and Pneumocystis pneumonia among homosexual men- New York City and California. MMWR Morb Mortal Wkly Rep. 1981;30:305–308. - PubMed
-
- Gottlieb GJ, et al. A preliminary communication on extensively disseminated Kaposi’s sarcoma in young homosexual men. Am J Dermatopathol. 1981;3:111–114. - PubMed
-
- Barre-Sinoussi F, et al. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS) Science. 1983;220:868–871. - PubMed
-
- Gelmann EP, et al. Proviral DNA of a retrovirus, human T-cell leukemia virus, in two patients with AIDS. Science. 1983;220:862–865. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

