A multi-step process of viral adaptation to a mutagenic nucleoside analogue by modulation of transition types leads to extinction-escape
- PMID: 20865120
- PMCID: PMC2928812
- DOI: 10.1371/journal.ppat.1001072
A multi-step process of viral adaptation to a mutagenic nucleoside analogue by modulation of transition types leads to extinction-escape
Abstract
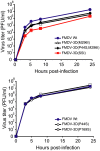
Resistance of viruses to mutagenic agents is an important problem for the development of lethal mutagenesis as an antiviral strategy. Previous studies with RNA viruses have documented that resistance to the mutagenic nucleoside analogue ribavirin (1-β-D-ribofuranosyl-1-H-1,2,4-triazole-3-carboxamide) is mediated by amino acid substitutions in the viral polymerase that either increase the general template copying fidelity of the enzyme or decrease the incorporation of ribavirin into RNA. Here we describe experiments that show that replication of the important picornavirus pathogen foot-and-mouth disease virus (FMDV) in the presence of increasing concentrations of ribavirin results in the sequential incorporation of three amino acid substitutions (M296I, P44S and P169S) in the viral polymerase (3D). The main biological effect of these substitutions is to attenuate the consequences of the mutagenic activity of ribavirin -by avoiding the biased repertoire of transition mutations produced by this purine analogue-and to maintain the replicative fitness of the virus which is able to escape extinction by ribavirin. This is achieved through alteration of the pairing behavior of ribavirin-triphosphate (RTP), as evidenced by in vitro polymerization assays with purified mutant 3Ds. Comparison of the three-dimensional structure of wild type and mutant polymerases suggests that the amino acid substitutions alter the position of the template RNA in the entry channel of the enzyme, thereby affecting nucleotide recognition. The results provide evidence of a new mechanism of resistance to a mutagenic nucleoside analogue which allows the virus to maintain a balance among mutation types introduced into progeny genomes during replication under strong mutagenic pressure.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Domingo E. Quasispecies: Concepts and Implications for Virology. 2006. In Curr Top Microbiol Immunol Vol, 299.
-
- Scheidel LM, Durbin RK, Stollar V. Sindbis virus mutants resistant to mycophenolic acid and ribavirin. Virology. 1987;158:1–7. - PubMed
-
- Richman DD, editor. Antiviral Drug Resistance. New York: John Wiley and Sons Inc; 1996.
-
- Domingo E. RNA virus evolution and the control of viral disease. Prog Drug Res. 1989;33:93–133. - PubMed
-
- Domingo E. Quasispecies and the development of new antiviral strategies. Progress in Drug Res. 2003;60:133–158. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

