Factors Contributing to Aromatic Stacking in Water: Evaluation in the Context of DNA
- PMID: 20865137
- PMCID: PMC2943206
- DOI: 10.1021/ja9934854
Factors Contributing to Aromatic Stacking in Water: Evaluation in the Context of DNA
Abstract
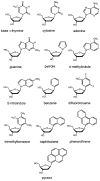
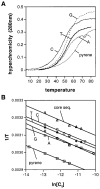
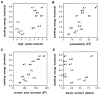
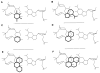
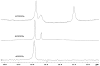
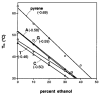
We report the use of thermodynamic measurements in a self-complementary DNA duplex (5'-dXCGCGCG)(2), where X is an unpaired natural or nonnatural deoxynucleoside, to study the forces that stabilize aqueous aromatic stacking in the context of DNA. Thermal denaturation experiments show that the core duplex (lacking X) is formed with a free energy (37 °C) of -8.1 kcal·mol(-1) in a pH 7.0 buffer containing 1 M Na(+). We studied the effects of adding single dangling nucleosides (X) where the aromatic "base" is adenine, guanine, thymine, cytosine, pyrrole, benzene, 4-methylindole, 5-nitroindole, trimethylbenzene, difluorotoluene, naphthalene, phenanthrene, and pyrene. Adding these dangling residues is found to stabilize the duplex by an additional -0.8 to -3.4 kcal·mol(-1). At 5 μM DNA concentration, T(m) values range from 41.7 °C (core sequence) to 64.1 °C (with dangling pyrene residues). For the four natural bases, the order of stacking ability is A > G ≥ T = C. The nonpolar analogues stack more strongly in general than the more polar natural bases. The stacking geometry was confirmed in two cases (X = adenine and pyrene) by 2-D NOESY experiments. Also studied is the effect of ethanol cosolvent on the stacking of natural bases and pyrene. Stacking abilities were compared to calculated values for hydrophobicity, dipole moment, polarizability, and surface area. In general, hydrophobic effects are found to be larger than other effects stabilizing stacking (electrostatic effects, dispersion forces); however, the natural DNA bases are found to be less dependent on hydrophobic effects than are the more nonpolar compounds. The results also point out strategies for the design nucleoside analogues that stack considerably more strongly than the natural bases; such compounds may be useful in stabilizing designed DNA structures and complexes.
Figures
References
-
- Pimentel GC, McClellan AL. The Hydrogen Bond. W H Freeman; San Francisco: 1960. pp. 206–225.
-
- Gould IR, Kollman PR. J Am Chem Soc. 1994;116:2493–2499.
-
- Cantor CR, Schimmel PR. Biophysical Chemistry Part III: The Behavior of Biological Macromolecules. Vol. 3. W H Freeman; San Francisco: 1980. pp. 1117–1133.
-
- Petersheim M, Turner DH. Biochemistry. 1983;22:256–263. - PubMed
-
- Turner DH, Sugimoto N, Freier SM. Annu Rev Biophys Biophys Chem. 1988;17:167–192. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
