Nonlinear gap junctions enable long-distance propagation of pulsating calcium waves in astrocyte networks
- PMID: 20865153
- PMCID: PMC2928752
- DOI: 10.1371/journal.pcbi.1000909
Nonlinear gap junctions enable long-distance propagation of pulsating calcium waves in astrocyte networks
Abstract
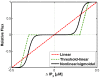
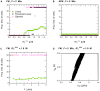
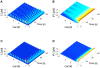
A new paradigm has recently emerged in brain science whereby communications between glial cells and neuron-glia interactions should be considered together with neurons and their networks to understand higher brain functions. In particular, astrocytes, the main type of glial cells in the cortex, have been shown to communicate with neurons and with each other. They are thought to form a gap-junction-coupled syncytium supporting cell-cell communication via propagating Ca(2+) waves. An identified mode of propagation is based on cytoplasm-to-cytoplasm transport of inositol trisphosphate (IP(3)) through gap junctions that locally trigger Ca(2+) pulses via IP(3)-dependent Ca(2+)-induced Ca(2+) release. It is, however, currently unknown whether this intracellular route is able to support the propagation of long-distance regenerative Ca(2+) waves or is restricted to short-distance signaling. Furthermore, the influence of the intracellular signaling dynamics on intercellular propagation remains to be understood. In this work, we propose a model of the gap-junctional route for intercellular Ca(2+) wave propagation in astrocytes. Our model yields two major predictions. First, we show that long-distance regenerative signaling requires nonlinear coupling in the gap junctions. Second, we show that even with nonlinear gap junctions, long-distance regenerative signaling is favored when the internal Ca(2+) dynamics implements frequency modulation-encoding oscillations with pulsating dynamics, while amplitude modulation-encoding dynamics tends to restrict the propagation range. As a result, spatially heterogeneous molecular properties and/or weak couplings are shown to give rise to rich spatiotemporal dynamics that support complex propagation behaviors. These results shed new light on the mechanisms implicated in the propagation of Ca(2+) waves across astrocytes and the precise conditions under which glial cells may participate in information processing in the brain.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Allen NJ, Barres BA. Glia more than just brain glue. Nature. 2009;457:675–677. - PubMed
-
- Volterra A, Meldolesi J. Astrocytes, from brain glue to communication elements: the revolution continues. Nat Rev Neurosci. 2005;6:626–640. - PubMed
-
- Santello M, Volterra A. Synaptic modulation by astrocytes via Ca2+dependent glutamate release. Neuroscience. 2009;158:253–259. - PubMed
-
- Bezzi P, Volterra A. A neuronglia signalling network in the active brain. Curr Opin Neurobiol. 2001;11:387–394. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

