Disruption of the lipid-transporting LdMT-LdRos3 complex in Leishmania donovani affects membrane lipid asymmetry but not host cell invasion
- PMID: 20865154
- PMCID: PMC2928753
- DOI: 10.1371/journal.pone.0012443
Disruption of the lipid-transporting LdMT-LdRos3 complex in Leishmania donovani affects membrane lipid asymmetry but not host cell invasion
Abstract
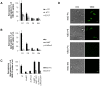
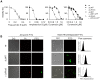
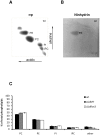
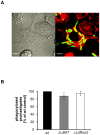
Maintenance and regulation of the asymmetric lipid distribution across eukaryotic plasma membranes is governed by the concerted action of specific membrane proteins controlling lipid movement across the bilayer. Here, we show that the miltefosine transporter (LdMT), a member of the P4-ATPase subfamily in Leishmania donovani, and the Cdc50-like protein LdRos3 form a stable complex that plays an essential role in maintaining phospholipid asymmetry in the parasite plasma membrane. Loss of either LdMT or LdRos3 abolishes ATP-dependent transport of NBD-labelled phosphatidylethanolamine (PE) and phosphatidylcholine from the outer to the inner plasma membrane leaflet and results in an increased cell surface exposure of endogenous PE. We also find that promastigotes of L. donovani lack any detectable amount of phosphatidylserine (PS) but retain their infectivity in THP-1-derived macrophages. Likewise, infectivity was unchanged for parasites without LdMT-LdRos3 complexes. We conclude that exposure of PS and PE to the exoplasmic leaflet is not crucial for the infectivity of L. donovani promastigotes.
Conflict of interest statement
Figures

Similar articles
-
Non-Vesicular Lipid Transport Machinery in Leishmania donovani: Functional Implications in Host-Parasite Interaction.Int J Mol Sci. 2023 Jun 26;24(13):10637. doi: 10.3390/ijms241310637. Int J Mol Sci. 2023. PMID: 37445815 Free PMC article. Review.
-
Phospholipid translocation and miltefosine potency require both L. donovani miltefosine transporter and the new protein LdRos3 in Leishmania parasites.J Biol Chem. 2006 Aug 18;281(33):23766-75. doi: 10.1074/jbc.M605214200. Epub 2006 Jun 19. J Biol Chem. 2006. PMID: 16785229
-
Functional cloning of the miltefosine transporter. A novel P-type phospholipid translocase from Leishmania involved in drug resistance.J Biol Chem. 2003 Dec 12;278(50):49965-71. doi: 10.1074/jbc.M308352200. Epub 2003 Sep 27. J Biol Chem. 2003. PMID: 14514670
-
Inactivation of the miltefosine transporter, LdMT, causes miltefosine resistance that is conferred to the amastigote stage of Leishmania donovani and persists in vivo.Int J Antimicrob Agents. 2007 Sep;30(3):229-35. doi: 10.1016/j.ijantimicag.2007.05.007. Epub 2007 Jul 12. Int J Antimicrob Agents. 2007. PMID: 17628445
-
Functions of phospholipid flippases.J Biochem. 2011 Feb;149(2):131-43. doi: 10.1093/jb/mvq140. Epub 2010 Dec 5. J Biochem. 2011. PMID: 21134888 Review.
Cited by
-
A functional genomic screen in Saccharomyces cerevisiae reveals divergent mechanisms of resistance to different alkylphosphocholine chemotherapeutic agents.G3 (Bethesda). 2021 Sep 27;11(10):jkab233. doi: 10.1093/g3journal/jkab233. G3 (Bethesda). 2021. PMID: 34568930 Free PMC article.
-
Non-Vesicular Lipid Transport Machinery in Leishmania donovani: Functional Implications in Host-Parasite Interaction.Int J Mol Sci. 2023 Jun 26;24(13):10637. doi: 10.3390/ijms241310637. Int J Mol Sci. 2023. PMID: 37445815 Free PMC article. Review.
-
Aminoglycerophospholipid flipping and P4-ATPases in Toxoplasma gondii.J Biol Chem. 2021 Jan-Jun;296:100315. doi: 10.1016/j.jbc.2021.100315. Epub 2021 Jan 21. J Biol Chem. 2021. PMID: 33485966 Free PMC article.
-
Investigation of the pathways related to intrinsic miltefosine tolerance in Leishmania (Viannia) braziliensis clinical isolates reveals differences in drug uptake.Int J Parasitol Drugs Drug Resist. 2019 Dec;11:139-147. doi: 10.1016/j.ijpddr.2019.02.005. Epub 2019 Feb 25. Int J Parasitol Drugs Drug Resist. 2019. PMID: 30850347 Free PMC article.
-
Phosphatidylserine exposure on the surface of Leishmania amazonensis amastigotes modulates in vivo infection and dendritic cell function.Parasite Immunol. 2013 Mar-Apr;35(3-4):109-119. doi: 10.1111/pim.12019. Parasite Immunol. 2013. PMID: 23163958 Free PMC article.
References
-
- Devaux PF. Static and dynamic lipid asymmetry in cell membranes. Biochemistry. 1991;30:1163–1173. - PubMed
-
- Fadeel B, Xue D. The ins and outs of phospholipid asymmetry in the plasma membrane: roles in health and disease. Crit Rev Biochem Mol Biol. 2009;44:264–277. Available: http://dx.doi.org/10.1080/10409230903193307 via the Internet. Accessed 7 Aug 2010. - DOI - PMC - PubMed
-
- de Freitas Balanco JM, Moreira ME, Bonomo A, Bozza PT, Amarante-Mendes G, et al. Apoptotic mimicry by an obligate intracellular parasite downregulates macrophage microbicidal activity. Curr Biol. 2001;11:1870–1873. - PubMed
-
- Wanderley JLM, da Silva LHP, Deolindo P, Soong L, Borges VM, et al. Cooperation between apoptotic and viable metacyclics enhances the pathogenesis of Leishmaniasis. PLoS One. 2009;4:e5733. Available: http://dx.doi.org/10.1371/journal.pone.0005733 via the Internet Accessed 7 Aug 2010. - DOI - PMC - PubMed
-
- Wanderley JLM, Moreira MEC, Benjamin A, Bonomo AC, Barcinski MA. Mimicry of apoptotic cells by exposing phosphatidylserine participates in the establishment of amastigotes of Leishmania (L) amazonensis in mammalian hosts. J Immunol. 2006;176:1834–1839. - PubMed

