Discordant behavioral effects of psychotomimetic drugs in mice with altered NMDA receptor function
- PMID: 20865248
- PMCID: PMC4818544
- DOI: 10.1007/s00213-010-2023-4
Discordant behavioral effects of psychotomimetic drugs in mice with altered NMDA receptor function
Abstract
Rationale: Enhancement of N-methyl-D: -aspartate receptor (NMDAR) activity through its glycine modulatory site (GMS) is a novel therapeutic approach in schizophrenia. Brain concentrations of endogenous GMS agonist D: -serine and antagonist N-acetyl-aspartylglutamate are regulated by serine racemase (SR) and glutamic acid decarboxylase 2 (GCP2), respectively. Using mice genetically, under-expressing these enzymes may clarify the role of NMDAR-mediated neurotransmission in schizophrenia.
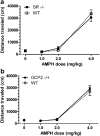
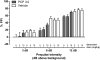
Objectives: We investigated the behavioral effects of two psychotomimetic drugs, the noncompetitive NMDAR antagonist, phencyclidine (PCP; 0, 1.0, 3.0, or 6.0 mg/kg), and the indirect dopamine receptor agonist, amphetamine (AMPH; 0, 1.0, 2.0, or 4.0 mg/kg), in SR -/- and GCP2 -/+ mice. Outcome measures were locomotor activity and prepulse inhibition (PPI) of the acoustic startle reflex. Acute effects of an exogenous GMS antagonist, gavestinel (0, 3.0, or 10.0 mg/kg), on PCP-induced behaviors were examined in wild-type mice for comparison to the mutants with reduced GMS activity.
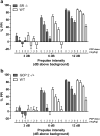
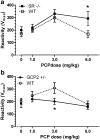
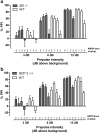
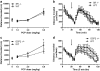
Results: PCP-induced hyperactivity was increased in GCP2 -/+ mice, and PCP-enhanced startle reactivity was increased in SR -/- mice. PCP disruption of PPI was unaffected in either mutant. In contrast, gavestinel attenuated PCP-induced PPI disruption without effect on baseline PPI or locomotor activity. AMPH effects were similar to controls in both mutant strains.
Conclusions: The results of the PCP experiments demonstrate that convergence of pharmacological and genetic manipulations at NMDARs may confound the predictive validity of these preclinical assays for the effects of GMS activation in schizophrenia. The AMPH data provide additional evidence that hyperdopaminergia in schizophrenia may be distinct from NMDAR hypofunction.
Figures
Similar articles
-
Failure of haloperidol to block the effects of phencyclidine and dizocilpine on prepulse inhibition of startle.Biol Psychiatry. 1991 Sep 15;30(6):557-66. doi: 10.1016/0006-3223(91)90025-h. Biol Psychiatry. 1991. PMID: 1834231
-
Metabotropic glutamate subtype 5 receptors modulate locomotor activity and sensorimotor gating in rodents.J Pharmacol Exp Ther. 2003 Jul;306(1):116-23. doi: 10.1124/jpet.103.048702. Epub 2003 Mar 26. J Pharmacol Exp Ther. 2003. PMID: 12660307
-
Blockade of growth hormone secretagogue receptor 1A signaling by JMV 2959 attenuates the NMDAR antagonist, phencyclidine-induced impairments in prepulse inhibition.Psychopharmacology (Berl). 2015 Dec;232(23):4285-92. doi: 10.1007/s00213-015-4054-3. Epub 2015 Aug 29. Psychopharmacology (Berl). 2015. PMID: 26319159 Free PMC article.
-
Directly and Indirectly Targeting the Glycine Modulatory Site to Modulate NMDA Receptor Function to Address Unmet Medical Needs of Patients With Schizophrenia.Front Psychiatry. 2021 Oct 1;12:742058. doi: 10.3389/fpsyt.2021.742058. eCollection 2021. Front Psychiatry. 2021. PMID: 34658976 Free PMC article. Review.
-
The Role of Serine Racemase in the Pathophysiology of Brain Disorders.Adv Pharmacol. 2018;82:35-56. doi: 10.1016/bs.apha.2017.10.002. Epub 2017 Nov 29. Adv Pharmacol. 2018. PMID: 29413527 Free PMC article. Review.
Cited by
-
A New Three-Hit Mouse Model of Neurodevelopmental Disorder with Cognitive Impairments and Persistent Sociability Deficits.Brain Sci. 2024 Dec 20;14(12):1281. doi: 10.3390/brainsci14121281. Brain Sci. 2024. PMID: 39766480 Free PMC article.
-
Availability of N-Methyl-d-Aspartate Receptor Coagonists Affects Cocaine-Induced Conditioned Place Preference and Locomotor Sensitization: Implications for Comorbid Schizophrenia and Substance Abuse.J Pharmacol Exp Ther. 2015 Jun;353(3):465-70. doi: 10.1124/jpet.115.223099. Epub 2015 Mar 18. J Pharmacol Exp Ther. 2015. PMID: 25788713 Free PMC article.
-
Genetic models of sensorimotor gating: relevance to neuropsychiatric disorders.Curr Top Behav Neurosci. 2012;12:251-318. doi: 10.1007/7854_2011_195. Curr Top Behav Neurosci. 2012. PMID: 22367921 Free PMC article. Review.
-
Failure of NMDA receptor hypofunction to induce a pathological reduction in PV-positive GABAergic cell markers.Neurosci Lett. 2011 Jan 25;488(3):267-71. doi: 10.1016/j.neulet.2010.11.043. Epub 2010 Nov 19. Neurosci Lett. 2011. PMID: 21094213 Free PMC article.
-
Quinoline Quest: Kynurenic Acid Strategies for Next-Generation Therapeutics via Rational Drug Design.Pharmaceuticals (Basel). 2025 Apr 22;18(5):607. doi: 10.3390/ph18050607. Pharmaceuticals (Basel). 2025. PMID: 40430428 Free PMC article. Review.
References
-
- Abi-Dargham A, van de Giessen E, Slifstein M, Kegeles LS, Laruelle M. Baseline and amphetamine-stimulated dopamine activity are related in drug-naïve schizophrenic subjects. Biol Psychiatry. 2009;65(12):1091–1093. - PubMed
-
- Allen NC, Bagade S, McQueen MB, Ioannidis JP, Kavvoura FK, Khoury MJ, Tanzi RE, Bertram L. Systematic meta-analyses and field synopsis of genetic association studies in schizophrenia: the SzGene database. Nat Genet. 2008;40(7):827–834. - PubMed
-
- Ballard TM, Pauly-Evers M, Higgins GA, Ouagazzal AM, Mutel V, Borroni E, Kemp JA, Bluethmann H, Kew JN. Severe impairment of NMDA receptor function in mice carrying targeted point mutations in the glycine binding site results in drug-resistant nonhabituating hyperactivity. J Neurosci. 2002;22(15):6713–6723. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

