Autophagy and regulation of lipid metabolism
- PMID: 20865370
- PMCID: PMC4052896
- DOI: 10.1007/978-3-642-14426-4_4
Autophagy and regulation of lipid metabolism
Abstract
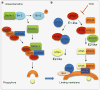
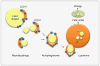
Macroautophagy (henceforth referred to as autophagy) is an in-bulk lysosomal degradative pathway that plays a crucial role in the maintenance of cellular homeostasis through the removal of damaged proteins and aged organelles. Following nutrient deprivation, a primary cellular response is the induction of autophagy that breaks down redundant cellular components and provides amino acids and additional precursor molecules for processes critical for cellular survival. In parallel, nutrient depletion leads to the mobilization of cellular lipid stores to supply free fatty acids for energy, thus pointing to regulatory and functional similarities between autophagy and lipid metabolism. The current chapter discusses the novel and mutually exclusive roles of autophagy in the regulation of lipid metabolism in the liver and of fat storage within the adipose tissue. Our studies in cultured hepatocytes and the murine liver have demonstrated that autophagy serves to degrade intracellular lipid stores through a process that we have termed "macrolipophagy" and that ablation of liver-specific autophagy leads to excessive hepatic lipid accumulation and the development of fatty liver. In contrast, preadipocytes in culture that lacked autophagy failed to differentiate into mature adipocytes and exhibited a reduction in fat storage that translated to decreased adipose tissue mass in an in vivo mouse model. These recent findings establish an association between autophagy and regulation of hepatic lipid metabolism and adipose tissue biology, thus providing new mechanistic insights into the regulation of these complex processes. These findings also highlight the possibility of novel therapeutic approaches, such as differential organ-specific regulation of autophagy to solve problems that arise from lipid over accumulation that occur in the metabolic syndrome and with aging.
Figures

References
-
- Beau I, Esclatine A, Codogno P. Lost to translation: when autophagy targets mature ribosomes. Trends Cell Biol. 2008;18:311–314. - PubMed
-
- Cuervo AM, Dice JF. A receptor for the selective uptake and degradation of proteins by lysosomes. Science. 1996;273:501–503. - PubMed
-
- Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114:842–845. - PubMed
-
- Esclatine A, Chaumorcel M, Codogno P. Macroautophagy signaling and regulation. Curr Top Microbiol Immunol. 2009;335:33–70. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

