The effect of acute exercise on glycogen synthesis rate in obese subjects studied by 13C MRS
- PMID: 20865425
- PMCID: PMC3019357
- DOI: 10.1007/s00421-010-1650-0
The effect of acute exercise on glycogen synthesis rate in obese subjects studied by 13C MRS
Abstract
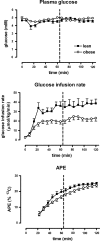
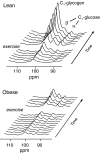
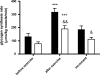
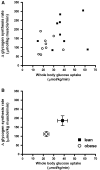
In obesity, insulin-stimulated glucose uptake in skeletal muscle is decreased. We investigated whether the stimulatory effect of acute exercise on glucose uptake and subsequent glycogen synthesis was normal. The study was performed on 18 healthy volunteers, 9 obese (BMI = 32.6 ± 1.2 kg/m(2), mean ± SEM) and 9 lean (BMI = 22.0 ± 0.9 kg/m(2)), matched for age and gender. All participants underwent a euglycemic hyperinsulinemic clamp, showing reduced glucose uptake in the obese group (P = 0.01), during which they performed a short intense local exercise (single-legged toe lifting). Dynamic glucose incorporation into glycogen in the gastrocnemius muscle before and after exercise was assessed by (13)C magnetic resonance spectroscopy combined with infusion of [1-(13)C]glucose. Blood flow was measured to investigate its potential contribution to glucose uptake. Before exercise, glycogen synthesis rate tended to be lower in obese subjects compared with lean (78 ± 14 vs. 132 ± 24 μmol/kg muscle/min; P = 0.07). Exercise induced highly significant rises in glycogen synthesis rates in both groups, but the increase in obese subjects was reduced compared with lean (112 ± 15 vs. 186 ± 27 μmol/kg muscle/min; P = 0.03), although the relative increase was similar (184 ± 35 vs. 202 ± 51%; P = 0.78). After exercise, blood flow increased equally in both groups, without a temporal relationship with the rate of glycogen synthesis. In conclusion, this study shows a stimulatory effect of a short bout of acute exercise on insulin-induced glycogen synthesis rate that is reduced in absolute values but similar in percentages in obese subjects. These results suggest a shared pathway between insulin- and exercise-induced glucose uptake and subsequent glycogen synthesis.
Figures

Similar articles
-
Magnetic resonance spectroscopy shows an inverse correlation between intramyocellular lipid content in human calf muscle and local glycogen synthesis rate.NMR Biomed. 2010 Feb;23(2):133-41. doi: 10.1002/nbm.1433. NMR Biomed. 2010. PMID: 19739109
-
Assessment of human muscle glycogen synthesis and total glucose content by in vivo 13C MRS.Eur J Clin Invest. 2000 Feb;30(2):122-8. doi: 10.1046/j.1365-2362.2000.00603.x. Eur J Clin Invest. 2000. PMID: 10651836
-
Quantitation of muscle glycogen synthesis in normal subjects and subjects with non-insulin-dependent diabetes by 13C nuclear magnetic resonance spectroscopy.N Engl J Med. 1990 Jan 25;322(4):223-8. doi: 10.1056/NEJM199001253220403. N Engl J Med. 1990. PMID: 2403659
-
Non-invasive studies of glycogen metabolism in human skeletal muscle using nuclear magnetic resonance spectroscopy.Curr Opin Clin Nutr Metab Care. 2001 Jul;4(4):261-6. doi: 10.1097/00075197-200107000-00003. Curr Opin Clin Nutr Metab Care. 2001. PMID: 11458018 Review.
-
Studies of gene expression and activity of hexokinase, phosphofructokinase and glycogen synthase in human skeletal muscle in states of altered insulin-stimulated glucose metabolism.Dan Med Bull. 1999 Feb;46(1):13-34. Dan Med Bull. 1999. PMID: 10081651 Review.
Cited by
-
Have guidelines addressing physical activity been established in nonalcoholic fatty liver disease?World J Gastroenterol. 2012 Dec 14;18(46):6790-800. doi: 10.3748/wjg.v18.i46.6790. World J Gastroenterol. 2012. PMID: 23239917 Free PMC article. Review.
-
Physical fitness in children with type 1 diabetes measured with six-minute walk test.Int J Endocrinol. 2013;2013:190454. doi: 10.1155/2013/190454. Epub 2013 Jun 27. Int J Endocrinol. 2013. PMID: 23935617 Free PMC article.
-
Assessment of Rapid Hepatic Glycogen Synthesis in Humans Using Dynamic 13C Magnetic Resonance Spectroscopy.Hepatol Commun. 2020 Jan 4;4(3):425-433. doi: 10.1002/hep4.1458. eCollection 2020 Mar. Hepatol Commun. 2020. PMID: 32140658 Free PMC article.
-
Changes in Skinfold Thicknesses and Body Fat in Ultra-endurance Cyclists.Asian J Sports Med. 2013 Mar;4(1):15-22. Epub 2012 Sep 29. Asian J Sports Med. 2013. PMID: 23785571 Free PMC article.
-
Effect of biological factors on successful measurements with skeletal-muscle (1)H-MRS.Ther Clin Risk Manag. 2016 Jul 20;12:1133-7. doi: 10.2147/TCRM.S84371. eCollection 2016. Ther Clin Risk Manag. 2016. PMID: 27499626 Free PMC article.
References
-
- Brozinick JT Jr, Etgen GJ Jr, Yaspelkis BB III, Ivy JL (1992) Contraction-activated glucose uptake is normal in insulin-resistant muscle of the obese Zucker rat. J Appl Physiol 73:382–387 - PubMed
-
- Cline GW, Petersen KF, Krssak M, Shen J, Hundal RS, Trajanoski Z, Inzucchi S, Dresner A, Rothman DL, Shulman GI. Impaired glucose transport as a cause of decreased insulin-stimulated muscle glycogen synthesis in type 2 diabetes. N Engl J Med. 1999;341:240–246. doi: 10.1056/NEJM199907223410404. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

