Lipoprotein receptors--an evolutionarily ancient multifunctional receptor family
- PMID: 20868222
- PMCID: PMC3529395
- DOI: 10.1515/BC.2010.129
Lipoprotein receptors--an evolutionarily ancient multifunctional receptor family
Abstract
The evolutionarily ancient low-density lipoprotein (LDL) receptor gene family represents a class of widely expressed cell surface receptors. Since the dawn of the first primitive multicellular organisms, several structurally and functionally distinct families of lipoprotein receptors have evolved. In accordance with the now obsolete 'one-gene-one-function' hypothesis, these cell surface receptors were originally perceived as mere transporters of lipoproteins, lipids, and nutrients or as scavenger receptors, which remove other kinds of macromolecules, such as proteases and protease inhibitors from the extracellular environment and the cell surface. This picture has since undergone a fundamental change. Experimental evidence has replaced the perception that these receptors serve merely as cargo transporters. Instead it is now clear that the transport of macromolecules is inseparably intertwined with the molecular machinery by which cells communicate with each other. Lipoprotein receptors are essentially sensors of the extracellular environment that participate in a wide range of physiological processes by physically interacting and coevolving with primary signal transducers as co-regulators. Furthermore, lipoprotein receptors modulate cellular trafficking and localization of the amyloid precursor protein (APP) and the β-amyloid peptide (Aβ), suggesting a role in the pathogenesis of Alzheimer's disease. Moreover, compelling evidence shows that LDL receptor family members are involved in tumor development and progression.
Figures
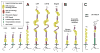

 and two
and two
 .
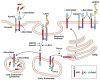
.
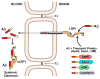
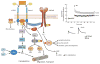


References
-
- Andersen OM, Yeung CH, Vorum H, Wellner M, Andreassen TK, et al. Essential role of the apolipoprotein E receptor-2 in sperm development. J Biol Chem. 2003;278:23989–95. - PubMed
-
- Arnaud L, Ballif BA, Forster E, Cooper JA. Fyn tyrosine kinase is a critical regulator of disabled-1 during brain development. Curr Biol. 2003;13:9–17. - PubMed
-
- Ashrafi K, Chang FY, Watts JL, Fraser AG, Kamath RS, et al. Genome-wide RNAi analysis of Caenorhabditis elegans fat regulatory genes. Nature. 2003;421:268–72. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
