Sociability and motor functions in Shank1 mutant mice
- PMID: 20868654
- PMCID: PMC3041833
- DOI: 10.1016/j.brainres.2010.09.026
Sociability and motor functions in Shank1 mutant mice
Abstract
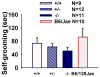
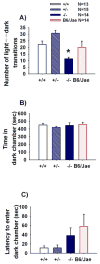
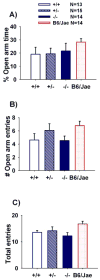
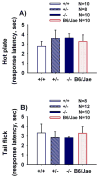
Autism is a neurodevelopmental disorder characterized by aberrant reciprocal social interactions, impaired communication, and repetitive behaviors. While the etiology remains unclear, strong evidence exists for a genetic component, and several synaptic genes have been implicated. SHANK genes encode a family of synaptic scaffolding proteins located postsynaptically on excitatory synapses. Mutations in SHANK genes have been detected in several autistic individuals. To understand the consequences of SHANK mutations relevant to the diagnostic and associated symptoms of autism, comprehensive behavioral phenotyping on a line of Shank1 mutant mice was conducted on multiple measures of social interactions, social olfaction, repetitive behaviors, anxiety-related behaviors, motor functions, and a series of control measures for physical abilities. Results from our comprehensive behavioral phenotyping battery indicated that adult Shank1 null mutant mice were similar to their wildtype and heterozygous littermates on standardized measures of general health, neurological reflexes and sensory skills. Motor functions were reduced in the null mutants on open field activity, rotarod, and wire hang, replicating and extending previous findings (Hung et al., 2008). A partial anxiety-like phenotype was detected in the null mutants in some components of the light ↔ dark task, as previously reported (Hung et al., 2008) but not in the elevated plus-maze. Juvenile reciprocal social interactions did not differ across genotypes. Interpretation of adult social approach was confounded by a lack of normal sociability in wildtype and heterozygous littermates. All genotypes were able to discriminate social odors on an olfactory habituation/dishabituation task. All genotypes displayed relatively high levels of repetitive self-grooming. Our findings support the interpretation that Shank1 null mice do not demonstrate autism-relevant social interaction deficits, but confirm and extend a role for Shank1 in motor functions.
Published by Elsevier B.V.
Figures





Similar articles
-
Repetitive behaviors in the Shank1 knockout mouse model for autism spectrum disorder: developmental aspects and effects of social context.J Neurosci Methods. 2014 Aug 30;234:92-100. doi: 10.1016/j.jneumeth.2014.05.003. Epub 2014 May 9. J Neurosci Methods. 2014. PMID: 24820912
-
Replicable in vivo physiological and behavioral phenotypes of the Shank3B null mutant mouse model of autism.Mol Autism. 2017 Jun 15;8:26. doi: 10.1186/s13229-017-0142-z. eCollection 2017. Mol Autism. 2017. PMID: 28638591 Free PMC article.
-
Communication impairments in mice lacking Shank1: reduced levels of ultrasonic vocalizations and scent marking behavior.PLoS One. 2011;6(6):e20631. doi: 10.1371/journal.pone.0020631. Epub 2011 Jun 9. PLoS One. 2011. PMID: 21695253 Free PMC article.
-
Mouse behavioral assays relevant to the symptoms of autism.Brain Pathol. 2007 Oct;17(4):448-59. doi: 10.1111/j.1750-3639.2007.00096.x. Brain Pathol. 2007. PMID: 17919130 Free PMC article. Review.
-
Modeling autism by SHANK gene mutations in mice.Neuron. 2013 Apr 10;78(1):8-27. doi: 10.1016/j.neuron.2013.03.016. Neuron. 2013. PMID: 23583105 Free PMC article. Review.
Cited by
-
The Role of Zinc and NMDA Receptors in Autism Spectrum Disorders.Pharmaceuticals (Basel). 2022 Dec 20;16(1):1. doi: 10.3390/ph16010001. Pharmaceuticals (Basel). 2022. PMID: 36678498 Free PMC article. Review.
-
Behavioral Evaluation of Angelman Syndrome Mice at Older Ages.Neuroscience. 2020 Oct 1;445:163-171. doi: 10.1016/j.neuroscience.2019.10.027. Epub 2019 Nov 12. Neuroscience. 2020. PMID: 31730795 Free PMC article.
-
Assessing behavioural and cognitive domains of autism spectrum disorders in rodents: current status and future perspectives.Psychopharmacology (Berl). 2014 Mar;231(6):1125-46. doi: 10.1007/s00213-013-3268-5. Epub 2013 Sep 19. Psychopharmacology (Berl). 2014. PMID: 24048469 Review.
-
S100B dysregulation during brain development affects synaptic SHANK protein networks via alteration of zinc homeostasis.Transl Psychiatry. 2021 Nov 5;11(1):562. doi: 10.1038/s41398-021-01694-z. Transl Psychiatry. 2021. PMID: 34741005 Free PMC article.
-
Behavioral Phenotyping for Autism Spectrum Disorders in Mice.Curr Protoc Toxicol. 2017 May 2;72:11.22.1-11.22.21. doi: 10.1002/cptx.19. Curr Protoc Toxicol. 2017. PMID: 28463420 Free PMC article.
References
-
- Abu-Elneel K, Liu T, Gazzaniga FS, Nishimura Y, Wall DP, Geschwind DH, Lao K, Kosik KS. Heterogeneous dysregulation of microRNAs across the autism spectrum. Neurogenetics. 2008;9:153–161. - PubMed
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fourth. Washington D.C: American Psychiatric Association; 1994.
-
- Berkel S, Marshall CR, Weiss B, Howe J, Roeth R, Moog U, Endris V, Roberts W, Szatmari P, Pinto D, Bonin M, Riess A, Engels H, Sprengel R, Scherer SW, Rappold GA. Mutations in the SHANK2 synaptic scaffolding gene in autism spectrum disorder and mental retardation. Nat Genet. 2010;42:489–491. - PubMed
-
- Blakeman KH, Hao JX, Xu XJ, Jacoby AS, Shine J, Crawley JN, Iismaa T, Wiesenfeld-Hallin Z. Hyperalgesia and increased neuropathic pain-like response in mice lacking galanin receptor 1 receptors. Neuroscience. 2003;117:221–227. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

