Casein kinase 2 regulates the NR2 subunit composition of synaptic NMDA receptors
- PMID: 20869595
- PMCID: PMC2947143
- DOI: 10.1016/j.neuron.2010.08.011
Casein kinase 2 regulates the NR2 subunit composition of synaptic NMDA receptors
Abstract
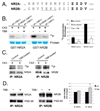
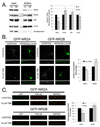
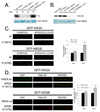
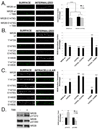
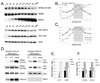
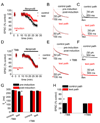
N-methyl-D-aspartate (NMDA) receptors (NMDARs) play a central role in development, synaptic plasticity, and neurological disease. NMDAR subunit composition defines their biophysical properties and downstream signaling. Casein kinase 2 (CK2) phosphorylates the NR2B subunit within its PDZ-binding domain; however, the consequences for NMDAR localization and function are unclear. Here we show that CK2 phosphorylation of NR2B regulates synaptic NR2B and NR2A in response to activity. We find that CK2 phosphorylates NR2B, but not NR2A, to drive NR2B-endocytosis and remove NR2B from synapses resulting in an increase in synaptic NR2A expression. During development there is an activity-dependent switch from NR2B to NR2A at cortical synapses. We observe an increase in CK2 expression and NR2B phosphorylation over this same critical period and show that the acute activity-dependent switch in NR2 subunit composition at developing hippocampal synapses requires CK2 activity. Thus, CK2 plays a central role in determining the NR2 subunit content of synaptic NMDARs.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Regulation of the NMDA receptor complex and trafficking by activity-dependent phosphorylation of the NR2B subunit PDZ ligand.J Neurosci. 2004 Nov 10;24(45):10248-59. doi: 10.1523/JNEUROSCI.0546-04.2004. J Neurosci. 2004. PMID: 15537897 Free PMC article.
-
Tyrosine phosphorylation regulates the endocytosis and surface expression of GluN3A-containing NMDA receptors.J Neurosci. 2013 Feb 27;33(9):4151-64. doi: 10.1523/JNEUROSCI.2721-12.2013. J Neurosci. 2013. PMID: 23447623 Free PMC article.
-
Phospho-regulation of synaptic and extrasynaptic N-methyl-d-aspartate receptors in adult hippocampal slices.Neuroscience. 2009 Feb 18;158(4):1446-59. doi: 10.1016/j.neuroscience.2008.11.006. Epub 2008 Nov 8. Neuroscience. 2009. PMID: 19041929
-
Regulation of NMDA receptor subunit expression and its implications for LTD, LTP, and metaplasticity.Neuropharmacology. 2008 Dec;55(7):1081-94. doi: 10.1016/j.neuropharm.2008.07.046. Epub 2008 Aug 8. Neuropharmacology. 2008. PMID: 18755202 Free PMC article. Review.
-
Regulation of glutamate receptors by striatal-enriched tyrosine phosphatase 61 (STEP61 ).J Physiol. 2021 Jan;599(2):443-451. doi: 10.1113/JP278703. Epub 2020 Apr 29. J Physiol. 2021. PMID: 32170729 Free PMC article. Review.
Cited by
-
Increased small conductance calcium-activated potassium type 2 channel-mediated negative feedback on N-methyl-D-aspartate receptors impairs synaptic plasticity following context-dependent sensitization to morphine.Biol Psychiatry. 2014 Jan 15;75(2):105-14. doi: 10.1016/j.biopsych.2013.04.026. Epub 2013 Jun 2. Biol Psychiatry. 2014. PMID: 23735878 Free PMC article.
-
Picrotoxin dramatically speeds the mammalian circadian clock independent of Cys-loop receptors.J Neurophysiol. 2013 Jul;110(1):103-8. doi: 10.1152/jn.00220.2013. Epub 2013 Apr 10. J Neurophysiol. 2013. PMID: 23576702 Free PMC article.
-
Rabphilin-3A undergoes phase separation to regulate GluN2A mobility and surface clustering.Nat Commun. 2023 Jan 24;14(1):379. doi: 10.1038/s41467-023-36046-6. Nat Commun. 2023. PMID: 36693856 Free PMC article.
-
NMDA receptor C-terminal signaling in development, plasticity, and disease.F1000Res. 2019 Aug 30;8:F1000 Faculty Rev-1547. doi: 10.12688/f1000research.19925.1. eCollection 2019. F1000Res. 2019. PMID: 31508206 Free PMC article. Review.
-
Short Tandem Repeat Variation in the CNR1 Gene Associated With Analgesic Requirements of Opioids in Postoperative Pain Management.Front Genet. 2022 Mar 3;13:815089. doi: 10.3389/fgene.2022.815089. eCollection 2022. Front Genet. 2022. PMID: 35360861 Free PMC article.
References
-
- Aksenova MV, Burbaeva GS, Kandror KV, Kapkov DV, Stepanov AS. The decreased level of casein kinase 2 in brain cortex of schizophrenic and Alzheimer's disease patients. FEBS letters. 1991;279:55–57. - PubMed
-
- Allende JE, Allende CC. Protein kinases. 4. Protein kinase CK2: an enzyme with multiple substrates and a puzzling regulation. Faseb J. 1995;9:313–323. - PubMed
-
- Alvestad RM, Grosshans DR, Coultrap SJ, Nakazawa T, Yamamoto T, Browning MD. Tyrosine dephosphorylation and ethanol inhibition of N-Methyl-D-aspartate receptor function. The Journal of biological chemistry. 2003;278:11020–11025. - PubMed
-
- Barria A, Malinow R. Subunit-specific NMDA receptor trafficking to synapses. Neuron. 2002;35:345–353. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

