The sigma-1 receptor chaperone as an inter-organelle signaling modulator
- PMID: 20869780
- PMCID: PMC2993063
- DOI: 10.1016/j.tips.2010.08.007
The sigma-1 receptor chaperone as an inter-organelle signaling modulator
Abstract
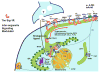
Inter-organelle signaling plays important roles in many physiological functions. Endoplasmic reticulum (ER)-mitochondrion signaling affects intramitochondrial calcium (Ca(2+)) homeostasis and cellular bioenergetics. ER-nucleus signaling attenuates ER stress. ER-plasma membrane signaling regulates cytosolic Ca(2+) homeostasis and ER-mitochondrion-plasma membrane signaling regulates hippocampal dendritic spine formation. Here, we propose that the sigma-1 receptor (Sig-1R), an ER chaperone protein, acts as an inter-organelle signaling modulator. Sig-1Rs normally reside at the ER-mitochondrion contact called the MAM (mitochondrion-associated ER membrane), where Sig-1Rs regulate ER-mitochondrion signaling and ER-nucleus crosstalk. When cells are stimulated by ligands or undergo prolonged stress, Sig-1Rs translocate from the MAM to the ER reticular network and plasmalemma/plasma membrane to regulate a variety of functional proteins, including ion channels, receptors and kinases. Thus, the Sig-1R serves as an inter-organelle signaling modulator locally at the MAM and remotely at the plasmalemma/plasma membrane. Many pharmacological/physiological effects of Sig-1Rs might relate to this unique action of Sig-1Rs.
Published by Elsevier Ltd.
Figures

References
-
- Su TP, et al. Steroid binding at sigma receptors suggest a link between endocrine, nervous, and immune systems. Science. 1988;240:219–221. - PubMed
-
- Snyder SH, Largent BL. Receptor mechanisms in antipsychotic drug action: focus on sigma receptors. J Neuropsychiatry Clin Neurosci. 1989;1:7–15. - PubMed
-
- Quirion R, et al. A proposal for the classification of sigma binding sites. Trend Pharmacol Sci. 1992;13:85–87. - PubMed
-
- Hayashi T, Su TP. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell. 2007;131:596–610. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DA-024442/DA/NIDA NIH HHS/United States
- MH-065503/MH/NIMH NIH HHS/United States
- F31 DA022932/DA/NIDA NIH HHS/United States
- R21 DA023397/DA/NIDA NIH HHS/United States
- R01 MH065503/MH/NIMH NIH HHS/United States
- R01 MH068212/MH/NIMH NIH HHS/United States
- F31DA-022932/DA/NIDA NIH HHS/United States
- DA-023397/DA/NIDA NIH HHS/United States
- T32 GM008688/GM/NIGMS NIH HHS/United States
- R01 DA024442/DA/NIDA NIH HHS/United States
- R01 DA020392/DA/NIDA NIH HHS/United States
- ZIA DA000206/ImNIH/Intramural NIH HHS/United States
- T32 GM08688/GM/NIGMS NIH HHS/United States
- MH-068212/MH/NIMH NIH HHS/United States
- DA-020392/DA/NIDA NIH HHS/United States
- Z99 DA999999/ImNIH/Intramural NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

