Remodeling of protein and mRNA expression in Leishmania mexicana induced by deletion of glucose transporter genes
- PMID: 20869991
- PMCID: PMC2974008
- DOI: 10.1016/j.molbiopara.2010.08.008
Remodeling of protein and mRNA expression in Leishmania mexicana induced by deletion of glucose transporter genes
Abstract
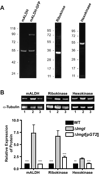
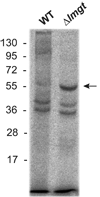
Glucose is a major nutrient in the insect vector stage of Leishmania parasites. Glucose transporter null mutants of Leishmania mexicana exhibit profound phenotypic changes in both insect stage promastigotes and mammalian host stage amastigotes that reside within phagolysosomes of host macrophages. Some of these phenotypic changes could be either mediated or attenuated by changes in gene expression that accompany deletion of the glucose transporter genes. To search for changes in protein expression, the profile of proteins detected on two-dimensional gels was compared for wild type and glucose transporter null mutant promastigotes. A total of 50 spots whose intensities changed significantly and consistently in multiple experiments were detected, suggesting that a cohort of proteins is altered in expression levels in the null mutant parasites. Following identification of proteins by mass spectrometry, 3 such regulated proteins were chosen for more detailed analysis: mitochondrial aldehyde dehydrogenase, ribokinase, and hexokinase. Immunoblots employing antisera against these enzymes confirmed that their levels were upregulated, both in glucose transporter null mutants and in wild type parasites starved for glucose. Quantitative reverse transcriptase PCR (qRT-PCR) revealed that the levels of mRNAs encoding these enzymes were also enhanced. Global expression profiling using microarrays revealed a limited number of additional changes, although the sensitivity of the microarrays to detect modest changes in amplitude was less than that of two-dimensional gels. Hence, there is likely to be a network of proteins whose expression levels are altered by genetic ablation of glucose transporters, and much of this regulation may be reflected by changes in the levels of the cognate mRNAs. Some of these changes in protein expression may reflect an adaptive response of the parasites to limitation of glucose.
Copyright © 2010 Elsevier B.V. All rights reserved.
Figures



References
-
- do Vale VF, Pereira MH, Gontijo NF. Midgut pH profile and protein digestion in the larvae of Lutzomyia longipalpis. J Insect Physiol. 2007;53:1151–1159. - PubMed
-
- Schlein Y. Sandfly diet and Leishmania. Parasitol. Today. 1986;2:175–177. - PubMed
-
- McConville MJ, de Souza D, Saunders E, Likic VA, Naderer T. Living in a phagolysosome; metabolism of Leishmania amastigotes. Trends Parasitol. 2007;23:368–375. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

