RNA interference against CFTR affects HL60-derived neutrophil microbicidal function
- PMID: 20870018
- PMCID: PMC3005861
- DOI: 10.1016/j.freeradbiomed.2010.09.012
RNA interference against CFTR affects HL60-derived neutrophil microbicidal function
Abstract
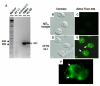
Biosynthesis of hypochlorous acid, a potent antimicrobial oxidant, in phagosomes is one of the chief mechanisms employed by polymorphonuclear neutrophils to combat infections. This reaction, catalyzed by myeloperoxidase, requires chloride anion (Cl(-)) as a substrate. Thus, Cl(-) availability is a rate-limiting factor that affects neutrophil microbicidal function. Our previous research demonstrated that defective CFTR, a cAMP-activated chloride channel, present in cystic fibrosis (CF) patients leads to deficient chloride transport to neutrophil phagosomes and impaired bacterial killing. To confirm this finding, here we used RNA interference against this chloride channel to abate CFTR expression in the neutrophil-like cells derived from HL60 cells, a promyelocytic leukemia cell line, with dimethyl sulfoxide. The resultant CFTR deficiency in the phagocytes compromised their bactericidal capability, thereby recapitulating the phenotype seen in CF patient cells. The results provide further evidence suggesting that CFTR plays an important role in phagocytic host defense.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures





References
-
- Klebanoff S. Myeloperoxidase: friend and foe. J Leukocyte Biol. 2005 May;77:598–625. - PubMed
-
- Nauseef W. How human neutrophils kill and degrade microbes: an integrated view. Immunol Rev. 2007;219:88–102. - PubMed
-
- Foote CS, Goyne TE, Lehrer RI. Assessment of chlorination by human neutrophils. Nature. 1983;301(5902):715–6. - PubMed
-
- Winterbourn CC, et al. Modeling the reactions of superoxide and myeloperoxidase in the neutrophil phagosome: implications for microbial killing. J Biol Chem. 2006;281(52):39860–9. - PubMed
-
- Murphy R, DeCoursey TE. Charge compensation during the phagocyte respiratory burst. Biochim Biophys Acta. 2006;1757(8):996–1011. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

