Combinations of physiologic estrogens with xenoestrogens alter ERK phosphorylation profiles in rat pituitary cells
- PMID: 20870566
- PMCID: PMC3018487
- DOI: 10.1289/ehp.1002512
Combinations of physiologic estrogens with xenoestrogens alter ERK phosphorylation profiles in rat pituitary cells
Abstract
Background: Estrogens are potent nongenomic phospho-activators of extracellular-signal-regulated kinases (ERKs). A major concern about the toxicity of xenoestrogens (XEs) is potential alteration of responses to physiologic estrogens when XEs are present simultaneously.
Objectives: We examined estrogen-induced ERK activation, comparing the abilities of structurally related XEs (alkylphenols and bisphenol A) to alter ERK responses induced by physiologic concentrations (1 nM) of estradiol (E2), estrone (E1), and estriol (E3).
Methods: We quantified hormone/mimetic-induced ERK phosphorylations in the GH3/B6/F10 rat pituitary cell line using a plate immunoassay, comparing effects with those on cell proliferation and by estrogen receptor subtype-selective ligands.
Results: Alone, these structurally related XEs activate ERKs in an oscillating temporal pattern similar (but not identical) to that with physiologic estrogens. The potency of all estrogens was similar (active between femtomolar and nanomolar concentrations). XEs potently disrupted physiologic estrogen signaling at low, environmentally relevant concentrations. Generally, XEs potentiated (at the lowest, subpicomolar concentrations) and attenuated (at the highest, picomolar to 100 nM concentrations) the actions of the physiologic estrogens. Some XEs showed pronounced nonmonotonic responses/inhibitions. The phosphorylated ERK and proliferative responses to receptor-selective ligands were only partially correlated.
Conclusions: XEs are both imperfect potent estrogens and endocrine disruptors; the more efficacious an XE, the more it disrupts actions of physiologic estrogens. This ability to disrupt physiologic estrogen signaling suggests that XEs may disturb normal functioning at life stages where actions of particular estrogens are important (e.g., development, reproductive cycling, pregnancy, menopause).
Figures

 , ✫). #p < 0.05 compared with cells treated with 1 nM E2, E1, or E3 alone.
, ✫). #p < 0.05 compared with cells treated with 1 nM E2, E1, or E3 alone.
 ). #p < 0.05 compared with cells treated with 1 nM E2, E1, or E3 alone.
). #p < 0.05 compared with cells treated with 1 nM E2, E1, or E3 alone.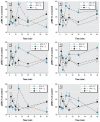
 , ✫). #p < 0.05 compared with cells treated with 1 nM E2, E1, or E3 alone.
, ✫). #p < 0.05 compared with cells treated with 1 nM E2, E1, or E3 alone.



Comment in
-
Estrogens from the outside in: alkylphenols, BPA disrupt ERK signaling in vitro.Environ Health Perspect. 2011 Jan;119(1):A34. doi: 10.1289/ehp.119-a34b. Environ Health Perspect. 2011. PMID: 21196144 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
