Conditional depletion of airway progenitor cells induces peribronchiolar fibrosis
- PMID: 20870756
- PMCID: PMC3056226
- DOI: 10.1164/rccm.201005-0744OC
Conditional depletion of airway progenitor cells induces peribronchiolar fibrosis
Abstract
Rationale: The respiratory epithelium has a remarkable capacity to respond to acute injury. In contrast, repeated epithelial injury is often associated with abnormal repair, inflammation, and fibrosis. There is increasing evidence that nonciliated epithelial cells play important roles in the repair of the bronchiolar epithelium after acute injury. Cellular processes underlying the repair and remodeling of the lung after chronic epithelial injury are poorly understood.
Objectives: To identify cell processes mediating epithelial regeneration and remodeling after acute and chronic Clara cell depletion.
Methods: A transgenic mouse model was generated to conditionally express diphtheria toxin A to ablate Clara cells in the adult lung. Epithelial regeneration and peribronchiolar fibrosis were assessed after acute and chronic Clara cell depletion.
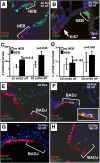
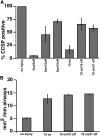
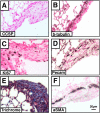
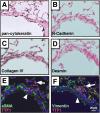
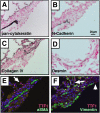
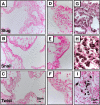
Measurements and main results: Acute Clara cell ablation caused squamous metaplasia of ciliated cells and induced proliferation of residual progenitor cells. Ciliated cells in the bronchioles and pro-surfactant protein C-expressing cells in the bronchiolar alveolar duct junctions did not proliferate. Epithelial cell proliferation occurred at multiple sites along the airways and was not selectively associated with regions around neuroepithelial bodies. Chronic Clara cell depletion resulted in ineffective repair and caused peribronchiolar fibrosis.
Conclusions: Colocalization of proliferation and cell type-specific markers demonstrate that Clara cells are critical airway progenitor cells. Continuous depletion of Clara cells resulted in persistent squamous metaplasia, lack of normal reepithelialization, and peribronchiolar fibrosis. Induction of proliferation in subepithelial fibroblasts supports the concept that chronic epithelial depletion caused peribronchiolar fibrosis.
Figures





References
-
- Van Winkle LS, Buckpitt AR, Nishio SJ, Isaac JM, Plopper CG. Cellular response in naphthalene-induced Clara cell injury and bronchiolar epithelial repair in mice. Am J Physiol 1995;269:L800–L818. - PubMed
-
- Reynolds SD, Hong KU, Giangreco A, Mango GW, Guron C, Morimoto Y, Stripp BR. Conditional Clara cell ablation reveals a self-renewing progenitor function of pulmonary neuroendocrine cells. Am J Physiol Lung Cell Mol Physiol 2000;278:L1256–L1263. - PubMed
-
- Evans MJ, Cabral-Anderson LJ, Freeman G. Role of the Clara cell in renewal of the bronchiolar epithelium. Lab Invest 1978;38:648–653. - PubMed
-
- Stripp BR, Sawaya PL, Luse DS, Wikenheiser KA, Wert SE, Huffman JA, Lattier DL, Singh G, Katyal SL, Whitsett JA. Cis-acting elements that confer lung epithelial cell expression of the CC10 gene. J Biol Chem 1992;267:14703–14712. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

