Transcranial direct current stimulation for the treatment of Parkinson's disease
- PMID: 20870863
- PMCID: PMC4162743
- DOI: 10.1136/jnnp.2009.202556
Transcranial direct current stimulation for the treatment of Parkinson's disease
Erratum in
- J Neurol Neurosurg Psychiatry. 2011 Mar;82(3):354
Abstract
Background: Progression of Parkinson's disease (PD) is characterised by motor deficits which eventually respond less to dopaminergic therapy and thus pose a therapeutic challenge. Deep brain stimulation has proven efficacy but carries risks and is not possible in all patients. Non-invasive brain stimulation has shown promising results and may provide a therapeutic alternative.
Objective: To investigate the efficacy of transcranial direct current stimulation (tDCS) in the treatment of PD.
Design: Randomised, double blind, sham controlled study.
Setting: Research institution.
Methods: The efficacy of anodal tDCS applied to the motor and prefrontal cortices was investigated in eight sessions over 2.5 weeks. Assessment over a 3 month period included timed tests of gait (primary outcome measure) and bradykinesia in the upper extremities, Unified Parkinson's Disease Rating Scale (UPDRS), Serial Reaction Time Task, Beck Depression Inventory, Health Survey and self-assessment of mobility.
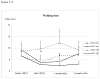
Results: Twenty-five PD patients were investigated, 13 receiving tDCS and 12 sham stimulation. tDCS improved gait by some measures for a short time and improved bradykinesia in both the on and off states for longer than 3 months. Changes in UPDRS, reaction time, physical and mental well being, and self-assessed mobility did not differ between the tDCS and sham interventions.
Conclusion: tDCS of the motor and prefrontal cortices may have therapeutic potential in PD but better stimulation parameters need to be established to make the technique clinically viable. This study was publicly registered (clinicaltrials.org: NCT00082342).
Figures


Comment in
-
Transcranial direct current stimulation as a treatment for Parkinson's disease--interesting, but not ready for prime time.J Neurol Neurosurg Psychiatry. 2010 Oct;81(10):1061. doi: 10.1136/jnnp.2010.205112. Epub 2010 Jun 19. J Neurol Neurosurg Psychiatry. 2010. PMID: 20562431 No abstract available.
References
-
- Braak H, Del Tredici K, Rub U, De Vos RAI, Steur ENHJ, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiology of Aging. 2003;24:197–211. - PubMed
-
- Elahi B, Elahi B, Chen R. Effect of Transcranial Magnetic Stimulation on Parkinson Motor Function-Systematic Review of Controlled Clinical Trials. Movement Disorders. 2009;24:357–63. - PubMed
-
- Priori A, Berardelli A, Rona S, Accornero N, Manfredi M. Polarization of the human motor cortex through the scalp. Neuroreport. 1998;9:2257–60. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
